- The Rayleigh refractometer utilises the refractive index of a gas to calculate its concentration.
- Thermal conductivity is used in katharometers. In these devices, the cooling of a wire causes a change in resistance proportional to gas concentration.
- Solubility is employed in devices such as rubber strips when an increase in length accompanies gas absorption.
- Light emission features in the Raman light scattering measurement device.
- Both infrared and ultraviolet absorption are used in gas concentration measurement.
Monthly Archives: July 2018
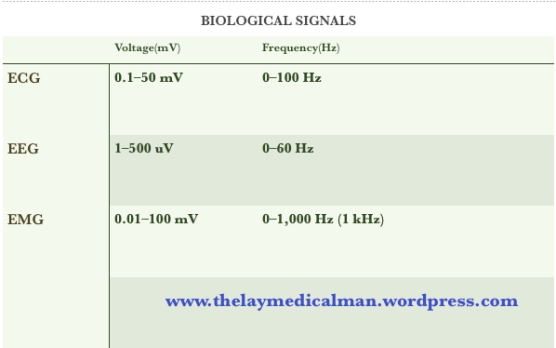
ECG – EEG – EMG Biological Signals
- The movement of ions across cell membranes during the depolarisation and repolarisation of myocytes and neurones generates electric potentials.
- Silver metal electrodes covered with a layer of silver chloride gel within an adhesive sponge pad can be used to measure these potentials at the skin.
- Ion movement near the electrode–skin interface induces movement of chloride ions within the gel layer. The ion concentration gradient promotes electron production at the electrode.
- A lead wire and voltmeter attached to the electrode allows measurement of the potential relative to a reference point. The reference point is usually a second skin electrode.
- Signals are then amplified, processed and displayed.
- Skeletal and cardiac muscles have higher amplitudes than cerebral neurones. This is because the amplitude of biological potentials is proportional to the number of simultaneously depolarising cells.
- The frequency of potentials is related to the fluctuating ion activity across cell membranes.
- Skeletal myocytes which undergo tetany have high frequencies of
up to 1 kHz. - Conversely, cardiac myocytes have lower frequencies due to their refractory periods
HUMIDITY AND ANESTHESIA
- Absolute humidity is the mass of water vapour present in a given volume of gas at
defined temperature and pressure (expressed as g of H2O/m3 of gas). - Relative humidity is the mass of water vapour present in a given volume of gas,
expressed as a percentage of the mass of water vapour required to saturate the same volume of gas at identical temperature and pressure. - The amount of water vapour required to saturate a known volume of gas increases with temperature, i.e. a gas saturated at 20°C contains less water than the same volume, saturated at 37°C
- Relative humidity (RH), can be calculated from the ratio of the mass of water vapour present (mP), to the mass required for satruation (mS) as
RH = mP/mS - If droplets are present, supersaturation has occurred and relative humidity exceeds 100%.
- From the gas laws, mass of a gas in a mixture is proportional to the partial pressure it exerts, thus: RH = water vapour pressure/ saturated water vapour pressure
- Instruments used to measure humidity are called hygrometers. Examples include:
Regnault’s hygrometer
Hair hygrometer
Wet and dry bulb thermometers
Humidity transducers
ELECTRICAL EQUIPMENT SAFETY
- Class 1 equipment is fully earthed.
- Class 2 equipment is double insulated.
- Class 3 is low voltage (24V).
PRESSURE & IT’S MEASUREMENT BY MANOMETER ⏲
💎Pressure = Force/Area
💎SI unit is Pascal
💎1Pa = 1N/m-2
💎It is the simplest method of pressure measurement
💎lt does not need calibration
💎So it can be used to calibrate other devices
💎The pressure is balanced against a column of liquid of known density – usually water for low pressures and mercury for higher pressures
💎The pressure is equal to the depth multiplied by the liquid density multiplied by the acceleration due to gravity; hence the commonly used units cm of H2O and mm of Hg
💎Mercury is 13 times more dense than water
💎The vertical height gives the pressure value
NEBULIZERS
- Aerosols are small particles of liquids or solids suspended in a carrying gas
- Medical aerosols can be produced by a nebulizer
- The therapeutic efficacy of the aerosol is dependent on the liquid or solid’s ability to remain in suspension and the depth reached by the aerosol on inhalation, and is dependent on its stability. These are both determined by the particle size.
- For liquid medication to enter the alveoli the droplets must be smaller than the diameter of the terminal bronchioles and fall within the size range of 0.005 µm to 50 µm in diameter.
- For droplet sizes below 5 µm, gravity exerts a negligible effect.
- Particles or droplets in the range 5 to 10 µm tend to deposit in the upper airways, with material below 5 µm penetrating further into the lungs.
- Below 3 µm, the droplets enter the alveoli and become therapeutically beneficial.
- Droplets below 1 µm are ideal; but if significantly smaller than this, the particles will be exhaled without having a therapeutic effect.
- The temperature for an aerosol generated by a nebulizer must not exceed 37°C and the process must not alter the structure of the medication being carried.
- This is the essential difference between vaporizers that generate a vapour and nebulizers that produce liquid droplets.
- Jet or gas driven nebulizer (atomizers)
- A high flow of gas is driven over a capillary tube that is immersed into the fluid to be nebulized. The high pressure air driven through the small orifice, generates negative pressure as a result of the Venturi effect. These nebulizers are simple and low cost, but small variations in gas flow rate can result in inconsistent delivery of aerosol to the patient.
- Ultrasound driven nebulizer
- The ultrasound nebulizer incorporates a ceramic piezoelectric transducer that changes electrical energy into mechanical energy (pressure oscillations). The transducer sits at the bottom of the chamber and vibrates at a frequency of 1.5 MHz. The vibrations are transmitted through the water. The diaphragm is in contact with the solution to be nebulized and violently shakes the solution into particles. At low frequencies, larger particles are produced, but at higher frequencies, a fine mist is generated
- Ultrasonic nebulizers tend to produce a more consistent particle size than jet nebulizers and, as a result, produce a much greater deposition into the lungs.
- But long-term use of ultrasonic nebulization might inadvertently affect surface tension stability in the alveoli
HUMIDIFIERS
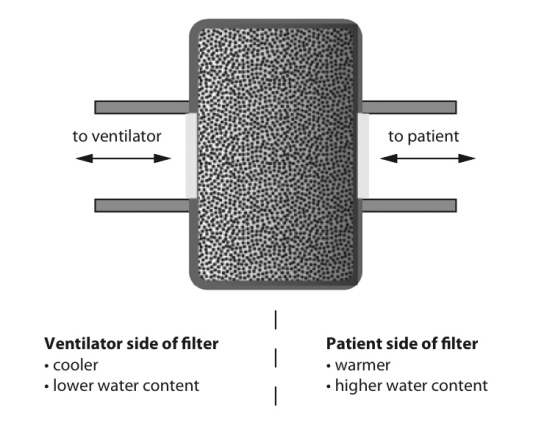
Heat and moisture exchange filter (HMEF)
- HMEF is inexpensive, disposable, passive and efficient enough to provide adequate humidification of dry gases for up to 24 hours.
- It creates a sealed unit, near the patient end of the breathing system, containing a hygroscopic material such as calcium chloride or silica gel.
- As the warm and moist gas from the patient reaches the HMEF, the moisture from the gas condenses onto the hygroscopic surface, simultaneously heating the element via the latent heat of condensation
- With the next inspiration of dry cold gas over the moist element this process is reversed, warming and humidifying the gas the patient receives.
- This process is about 80% efficient
- The addition of a 0.2 mm filter renders the interface impermeable to bacteria and viruses
- The disadvantages are that because it is a passive device, the HMEF is not 100% efficient and the patient will therefore lose heat and moisture over time, although this is negligible. Also, it adds dead space ranging from 8 mL in a paediatric HMEF to 100 mL in an adult, while the additional resistance can be up to 2.0 cm H2O ( may not create issues if respiratory function is not compromised significantly).
- The hygroscopic material and filter can act as a dam to secretions, greatly increasing the work of breathing. This is easily remedied by vigilance and replacement.
Water bath humidifiers
- Active water baths can achieve 100% efficiency and can also be used to heat the patient
- But they are bulky and complex and better suited for patients requiring longer-term ventilation or oxygen therapy.
- Passive water baths simply consist of a chamber of water through which the inspired gas is bubbled to achieve full saturation.
- The disadvantage is that the temperature of the water limits the maximum achievable humidity.
- Cooling of the water bath happens secondary to the latent heat of vaporization as the water is vaporized. This is remedied by an active system incorporating a heating element and thermostat.
- The system is designed to keep the water bath at a specific temperature (40–60°C). This increases the temperature of the gas mixture and therefore the achievable humidity. This system is capable of delivering fully saturated gas at 37°C at high flow rates which represents a significant advantage over the HMEF.
- All water baths need to include a water trap in their design, because the cooling of the gas as it moves away from the hot bath to the patient will result in condensation which can accumulate and could result in wet drowning. This risk may be minimized by heating the tubing and preventing condensation forming.
- Water baths at around 40°C minimize the risk of scalding the patient’s airways with overly heated gas, but run the risk of creating an ideal environment for microbial growth.
- By heating the water to 60°C the risk of bacterial contamination is reduced but the gas temperature must now be very carefully monitored.
- A thermistor on a feedback loop to the water bath’s thermostat can adjust the temperature of the water, and therefore inspired gas, to ensure that the patient does not suffer from airway scalding.
- The ideal size of water droplets for humidification is 5–10 microns. Smaller droplets will descend to the alveoli and larger ones will condense in the trachea.
- Scalding is a risk associated with water bath types when the temperature within exceeds 37C.
- Nebulisers are more efficient than water bath types of humidifiers. The Bernoulli effect describes the drop in pressure occurring at a jet, where velocity is greatest, which is employed to draw up water from a reservoir. This effect is used in spinning disc and gas driven humidifiers among others.
Latent Heat and its applications in anesthesia practice
- Heat capacity: The heat energy required to raise the temperature of a given object by one degree. (J.K−1 or J.°C−1)
- Specific heat capacity: The heat energy required to raise the temperature of one kilogram of a substance by one degree. (J.kg−1.K−1 or J.kg−1.°C−1)
- But not all heat energy results in a temperature change.
- Latent heat: This is the heat energy that is required for a material to undergo a change of phase. (J) The heat is not utilised for raising the temperature, but for changing the phase.
- If heat is applied to matter, temperature increases until the melting or boiling point is reached. At these points the addition of further heat energy is used to change the state of matter from solid to liquid and from liquid to gas. This does not cause a change in temperature. The energy required at these points is referred to as latent heat of fusion and latent heat of vaporisation, respectively.
- Specific latent heat is the heat required to convert one kilogram of a substance from one phase to another at a given temperature.
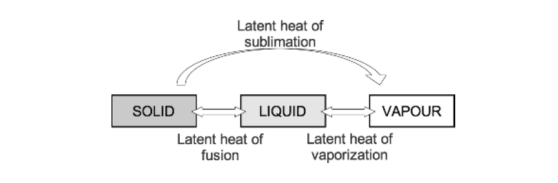
- As temperature increases, the amount of additional energy required to overcome the intermolecular forces of attraction falls until the critical temperature of a substance is reached. At this point the specific latent heat is zero, as no further energy is required to complete the change in state of the substance.
APPLICATIONS
- Variable bypass vaporisers function by passing a small amount of fresh gas through the vaporising chamber, which is fully saturated with anaesthetic vapour. This removes vapour from the chamber. Further vaporisation from the anaesthetic liquid must occur to replace the vapour removed, which requires energy from the latent heat of vaporisation. This cools the remaining liquid, reducing the saturated vapour pressure and thus the concentration of anaesthetic vapour delivered, resulting in an unreliable device.
- Temperature compensation features help to overcome this problem; a copper heat sink placed around the vaporising chamber is one such example. Copper has a high heat capacity and donates energy required for latent heat of vaporisation, maintaining a stable temperature and reliable delivery of anaesthetic agent.
- Evaporation of sweat is another example. It requires the latent heat of vaporisation, which is provided by the skin’s surface, exerting a cooling effect upon the body.
- Evaporation from open body cavities can be a cause of significant heat loss from patients while under anaesthesia.
- These principles are also applicable to blood transfusion. Blood is stored at 5°C and has a specific heat capacity of 3.5 kJ·kg−1·K−1. If cold blood were transfused into a patient without pre-warming, the heat energy required to warm the blood to body temperature would need to be supplied by the patient, which would have a significant cooling effect.
Ohm’s Law
- The strength of an electric current varies directly with the electromotive force (voltage) and inversely with the resistance. So I = V/R or V = IR where V is voltage, I is current and R is resistance.
- The equation can be used to calculate any of the above values when the other two are known. When R is calculated, it may represent resistance or impedance depending on the type of circuit being used (AC/DC)
- Resistance: The opposition to flow of direct current. (ohms, Ω)
- Reactance: The opposition to flow of alternating current. (ohms, Ω)
- Impedance: The total of the resistive and reactive components of opposition to electrical flow. (ohms, Ω)
- The reactance of an inductor is high and comes specifically from the back electromotive force that is generated within the coil. It is, therefore, difficult for AC to pass.
- The reactance of a capacitor is relatively low but its resistance can be high; therefore, direct current (DC) does not pass easily.
Thermistors and their use in anesthesia
- A thermistor is a temperature-sensitive resistor whose resistance changes with temperature.
- Most temperature-sensitive resistors are constructed from a semiconductor material (carefully chosen metal oxides) and the resistance increases with a fall in temperature (they have a negative temperature coefficient)
- So they are known as negative thermal conductivity (NTC) thermistors.
- A Wheatstone bridge circuit is used to measure the resistance accurately.
- The main disadvantage of thermistors is the non-linear resistance versus temperature characteristic, although this can be compensated for using an appropriate calibration equation programmed into an electronic measurement system.
- Thermistors remain highly popular due to their cost, miniature size and convenience.
- Thermistor probes are commonly placed in the nasopharynx, oesophagus, rectum or bladder (integrated with a urinary catheter).
- They have excellent accuracy and their small mass means that there is a quick response to variations in temperature.
- But they ‘age’ and their resistance changes with time. They also exhibit hysteresis.
- True or False? ‘A thermistor comprises a junction of dissimilar metals’
- Answer: False. Dissimilar junctional metals are thermocouples
- True or false: ‘A thermistor demonstrates the Seebeck effect’
- Answer: False. The Seebeck effect applies to themocouple
