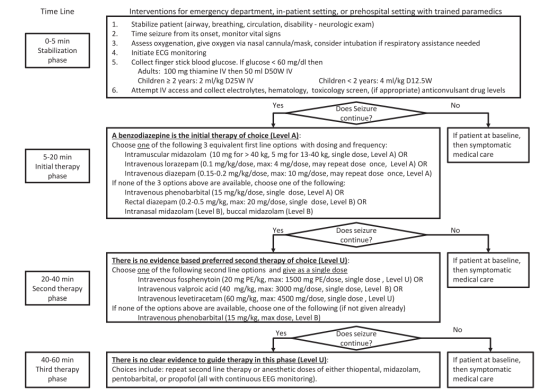
AMERICAN EPILEPSY SOCIETY GUIDELINES 2016: STATUS EPILEPTICUS



Incidence of overt Upper GI Bleed (UGIB) ranges from 1.5 to 8.5% of all ICU patients but may be as high as 15% if no prophylaxis is used.
RISK FACTORS
Mechanical ventilation >48 h
Coagulopathy – INR>1.5 or platelet count <50,000
Others: Shock, Sepsis, Hepatic failure,Acute Renal failure, Multiple trauma, Burns >35% of total body surface area, Organ transplantation, Head trauma, Spinal trauma, History of PUD or UGIB
SPECIFIC POINTS REGARDING TREATMENT
Thrombocytopenia can develop in neurosurgical patients on H2 Blockers
The use of H2Bs and PPIs may increase the frequency of nosocomial pneumonia.
PROPHYLAXIS IS RECOMMENDED FOR ICU PATIENTS WHO EXHIBIT:
Coagulopathy (platelet count < 50,000 per m 3 , INR > 1.5, partial thromboplastin time (PTT) >2 times the control value)
Mechanical ventilation >48 h
History of GI ulceration or bleeding within the past year
Two or more of the following risk factors: sepsis; ICU stay >1 week; occult GIB ≥6 days; glucocorticoid therapy (>250 mg hydrocortisone).
REASONS FOR UGIB IN ICU PATIENTS:
The glycoprotein mucous layer may be denuded by increased concentrations of refluxed bile salts or uremic toxins common in critically ill. Alternatively, or in addition, mucosal integrity may be compromised due to poor perfusion associated with shock, sepsis, and trauma.
Excessive gastrin stimulation of parietal cells has been detected in patients with head trauma as oppose to be normal or subnormal in most other ICU patients.
Systemic steroids double the risk of a new episode of UGIB or perforation. Concomitant use with high doses of NSAIDs has been associated with a 12-fold increased risk for upper GI complications.
Helicobacter pylori infection
EMPIRICAL THERAPY
Start with an IV bolus of 80 mg and continue IV infusion at 8 mg/h for a total of 72 h. If no signs of rebleeding after 24 h, switch to oral PPI.
Octreotide is used in variceal bleeding. Start with an IV bolus of 50 mcg and continue IV infusion at 50 mcg/h for 3–5 days.
UGIB IN HEAD INJURY & OTHER NEUROSURGICAL PATIENTS:
They are more prone for UGIB because of 1. Frequent use of systemic steroids 2. Increased gastrin secretion 3. Significant gastric intramucosal acidosis is common in severe head injury. 4. Primary insult to the central nervous system may result in derangement of splanchnic blood flow secondary to neurohumoral mechanisms.
In head injury, GI dysfunction also may manifest as gastroparesis, ileus, increased intestinal mucosal permeability
Plasma levels of cortisol and age are independent predictors of stress ulcers following acute head injury.
#GastroIntestinalBleed , #StressUlcer , #ICU , #Anesthesia , #CriticalCare, #IntensiveCare , #NeuroSurgery , #HeadInjury , #TBI,#NeuroCriticalCare
Reference: Gastrointestinal Hemorrhage in Neurosurgical Critical Care Meghan Bost, Kamila Vagnerova , Ch:84, Essentials of Neurosurgical Anesthesia & Critical Care 2012 Strategies for Prevention, Early Detection, and Successful Management of Perioperative Complications
Mannitol is a monosaccharide available as 10% & 20% solutions
DURING NEUROSURGERY/ IN NEUROCRITICAL CARE:
✔️Mannitol is freely filtered in the glomerulus but won’t get reabsorbed in the tubules; so it will drive water from the interstitium which gets eliminated as urine. Hence acts as an osmotic diuretic
✔️When blood brain barrier is intact, the osmotic gradient created by mannitol will move water from the cerebral extravascular compartment to the intravascular space, reducing ICP. If blood brain barrier is not intact, it will worsen cerebral edema.
✔️The expansion of the plasma volume caused by mannitol will reduce the viscosity and improve cerebrovascular microcirculation and oxygenation. The increase in cardiac output can also cause an increase in regional blood flow which will cause a compensatory cerebrovascular vasoconstriction in areas where autoregulation is intact.
IN CRUSH INJURY / MYOGLOBINURIA
✔️Will release renal prostaglandins, which will cause renal vasodilation and increase tubular urine flow causing a solute washout and avoidance of tubular obstruction #TheLayMedicalMan
MECHANISM BEHIND ADVERSE EFFECTS
✔️The initial increase in plasma volume as a result of drawing of water into the vascular component and the resultant increase in cardiac output can precipitate heart failure in cardiac patients
✔️The osmotic diuresis can cause hypernatremia [increases urinary losses of both sodium and electrolyte-free water] , metabolic acidosis and hyperosmolarity. It has been advised that therapy should be monitored and titrated so that osmolarity doesn’t go up beyond 300 mOsm/L
✔️The rise in the plasma potassium concentration following hypertonic mannitol is due to the movement of potassium out of the cells into the extracellular fluid as the rise in cell potassium concentration induced by water loss favors passive potassium exit through potassium channels in the cell membrane
✔️Though it has been used for renal protection, the reduction in renal perfusion resulting from hypovolemia caused by diuresis can adversely affect renal function; so should be avoided in patients with renal dysfunction
#Neuroanesthesia , #Anesthesia , #Neurology , #CriticalCare
🔸ICP data can be used to
✔️predict outcome and evolution of intracranial pathology
✔️calculate and manage cerebral perfusion pressure (CPP) [without an ICP monitor, CPP is not known].
✔️direct management strategies, and
✔️limit the use of potentially deleterious therapies.
🔸Cerebral herniation is a pressure issue and an ICP monitor may allow early detection; it is preferable to avoid herniation than to treat it
🔸Information from an ICP monitor may provide useful information to guide patient care. For example, a patient with a worrisome-appearing CT scan who does not have intracranial hypertension may not require the same degree of treatment as a patient with a similar scan but elevated ICP. Similarly, a patient with elevated ICP that is refractory to escalating management becomes an early candidate for “second tier” treatments or if very high, even withdrawal of care.
🔸ICP values have prognostic value and so it can guide management and discussions with the family about outcomes
🔸Even transient episodes of severely raised ICP and ischemia can be devastating to the traumatized brain, making it critical to accurately and continuously monitor ICP & CPP. Because insertion of intraparenchymal ICP monitors is safe, the ability to monitor CPP per se is a supportable argument for widespread ICP monitoring.
🔸Perhaps more important than a single ICP threshold may be a trend over time, ICP waveform analysis, or whether the ICP value is associated with other detrimental effects.
🔸When both ICP and brain oxygen are treated, the outcome may be better than if just ICP is treated after TBI
🔸The ICP waveform is a modified arterial pressure tracing
🔸 It has 3 peaks: P1, P2 & P3
🔸 P1 is a result of transmitted pressure from choroid plexus
🔸 The amplitude of P2 changes with brain compliance. If compliance is poor, amplitude will be high ( can even exceed that of P1) and vice versa
🔸P3 represents the dicrotic notch
🔸 Lundberg (A) or Plateau waves are steep rise of ICP to over 50 mm of Hg and lasting for 5-20 minutes; then it falls abruptly. Are Always pathological and indicates significantly reduced compliance
🔸 Lundberg (B) waves are oscillations occurring every 1-2 minutes where ICP rises to over 20-30 mm of Hg from baseline in a crescendo manner. They are supposed to be result of altered cerebral (B)lood volume and altered tone of cerebral (B)lood vessels
🔸 Lundberg (C) waves are oscillations whose amplitude is less than that of B waves and are supposed to result because of interactions between cardiac and respiratory (C)ycles. They occur also in healthy individuals
METHODS OF MEASUREMENT OF ICP
➿ Intraventricular catheter – ventriculostomy represents the “gold standard” for pressure measurement
✔️Normally placed in the frontal horn of lateral ventricle
✔️Allows therapeutic CSF drainage
✔️Creates a pathway for infection
✔️In case of the Integra Neuroscience external drainage catheter, ICP readings are based on a fluid-filled transduction system that transmits changes in ICP through a saline-filled tube to a diaphragm on a strain gauge transducer. This monitor must be leveled with the foramen of Monro (approximately the level of the external auditory canal) after insertion and should be zero-balanced daily. The level of the drain can be adjusted to allow more or less CSF drainage.
➿Subdural bolt / Catheters
✔️ less invasive
✔️ Bolts commonly use fiberoptic technology that allows continuous ICP monitoring without CSF drainage. The fiberoptic type of catheter can be placed in the subdural space or in the brain parenchyma
✔️ Usually subdural space over frontal lobe of non-dominant hemisphere is selected
✔️ Prone to signal damping and calibration drift
✔️ Potential risk of infection
✔️ Doesn’t require penetration of brain tissue
✔️Camino Post Craniotomy Subdural Pressure Monitor utilizes the craniotomy bur holes and flap as a point of entry. The monitor is zero-balanced and then tunneled under the scalp toward the craniotomy bur hole of choice and positioned in the subdural space. This monitor contains a microtransducer at the tip, which is similar to the OLM ICP monitor ( see below)
✔️Gaeltec ICT/B pressure sensor is intended to monitor ICP subdurally. It contains a balloon-covered pressure sensor that is activated when filled with air. This monitor is self–zero-balanced in vivo and is reusable.
➿Intracerebral transducer
✔️Parenchymal devices are easier to place, particularly when altered ventricular anatomy may limit ventricular catheter placement.
✔️However, intraparenchymal fiber-optic and electronic strain gauge systems are more expensive and cannot be recalibrated once in situ
✔️Inability to check zero calibration & drain CSF
✔️ Risk of infection
✔️Less reliable
✔️The Camino OLM ICP monitor measures ICP in the intraparenchymal tissue or subarachnoid space. It contains a transducer at the distal tip, thus measuring pressure without a fluid-filled system. The catheter is secured to the skull through an adjustable bolt, allowing placement at variable depths (up to 5 cm).
✔️The Codman Microsensor catheter can be used as an intraparenchymal or intraventricular monitor, depending on the depth of the catheter
✔️ Spiegelberg ICP monitors measure ICP through an air-pouch system attached to a pressure transducer connected to an electronic device. The probes differ, depending on where they rest (Epidural or Intraparenchymal)
🔸The incidence of infection ~ 2-7% with monitoring ≥ 5 days
🔸The risks are slightly greater with dural penetration
🔸The zero reference point of the transducer is usually taken as the external auditory meatus
🔸 Rather than the waveform type, the important factors appear to be the degree and duration of ICP elevation
🔸Two emerging non-invasive ICP monitoring methods include measuring the optic nerve sheath diameter (ONSD) as seen on an ultrasound probe placed on the superolateral aspect of the orbit and the pulsatility index (PI) which is cal- culated from transcranial Doppler studies (TCD).
#NeuroAnesthesia , #anaesthesia , #TheLayMedicalMan , #NeuroCriticalCare , #CriticalCare , #NeuroICU
📌Ictal bradycardia/asystole is a poorly recognised cause of collapse late in the course of a typical complex partial seizure
📌It is important to identify ictal bradycardia as a potential harbinger of lethal rhythms, such as asystole, as this may be one important mechanism leading to sudden unexpected death in epilepsy (SUDEP)
📌Tachycardia is the most common rhythm abnormality occurring in 64–100% of temporal lobe seizures. Ictal bradycardia has been reported in less than 6% of patients with complex partial seizures
📌The ictal bradycardia syndrome occurs in mostly in patients with temporal lobe seizures.
📌It is believed that abnormal neuronal activity during a seizure can affect central autonomic regulatory centres in the brain leading to cardiac rhythm changes.
📌Ictal bradycardia/asystole may be unrecognised until documented during video-electroencephalograph (video EEG)–electrocardiogram (ECG) monitoring in those with refractory epilepsy, often in the context of pre-surgical evaluation
📌Other rhythm abnormalities which can occur are change in heart rate variability, ictal tachycardias and atrioventricular (AV) block
📌If sufficiently severe, the ictal-induced bradyarrhythmia temporarily impairs both cerebral perfusion and cortical function; the result has the dual effect of terminating the seizure, while at the same time triggering syncope with consequent loss of consciousness and postural tone. In essence, a complex partial seizure patient may manifest both seizure and syncope features during the same episode.
📌There are currently no guidelines on who should undergo further cardiovascular investigations ; dual chamber pacemaker implantation has been suggested as a treatment in the long term, for epilepsy patients who manifest this syndrome and suffer repeated falls; but there is not much mention in literature both about diagnosis and about pharmacological and non pharmacological interventions to counter such episodes when presenting as an emergency situation in the perioperative scenario , especially when the patient is under anesthesia.
#Neurology , #NeuroCriticalCare , #Anesthesia , #LayMedicalMan , #CriticalCare , #Epilepsy , #Cardiology , #CardiacAnesthesia
Reference: Ictal bradycardia and atrioventricular block: a cardiac manifestation of epilepsy; Salman S. Allana Hanna N. Ahmed Keval Shah Annie F. Kelly, Oxford Medical Case Reports, British Journal of Cardiology : Ictal Bradycardia and Asystole Associated with Intractable Epilepsy: A Case Series Elijah Chaila, Jaspreet Bhangu, Sandya Tirupathi, Norman Delanty; Ictal Asystole-Life-Threatening Vagal Storm or a Benign Seizure Self-Termination Mechanism? David G. Benditt, Gert van Dijk, Roland D. Thijs (Editorial:Circulation )
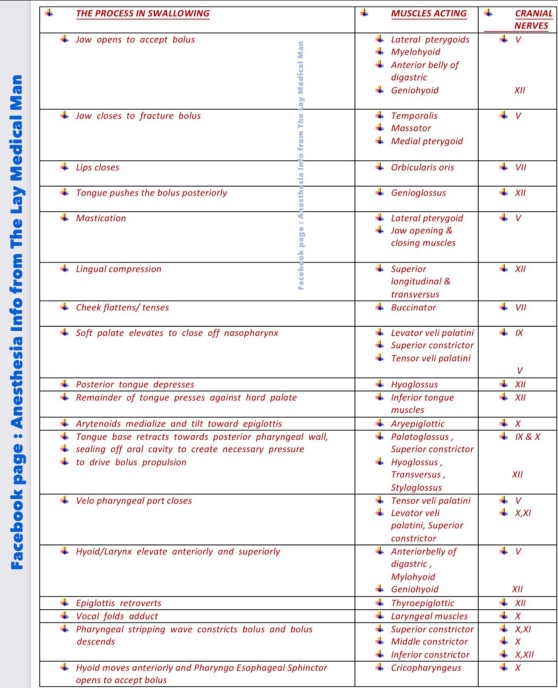 Cranial nerves V,VII,IX,X,XI,XII contributes
Cranial nerves V,VII,IX,X,XI,XII contributes