Category Archives: Physiology
DIFFERENCES BETWEEN THE TYPES OF HYPOXIA

HYPOXIC HYPOXIA (e.g. High Altitude)
- PaO2 is reduced ( as FiO2 is low)
- So more extraction of O2: So PvO2 is reduced
- Based on the above formula, both arterial and venous oxygen content also will be reduced
ANAEMIC HYPOXIA (e.g. haemorhhage)
- PaO2 will be normal
- But arterial oxygen content will be reduced due to lower values of Hb, as per the above formula reducing the oxygen delivery to tissues and increasing the cardiac work
- So there will be more oxygen extraction leading to a low PvO2 and venous oxygen content.
STAGNANT HYPOXIA (e.g. Cardiogenic shock)
- PaO2 is normal
- PvO2 is also normal
- There is no reduction in the arterial and venous oxygen content too.
- However, circulatory dysfunction results in inadequate oxygen delivery to organs
HISTOTOXIC HYPOXIA (e.g. Cyanide poisoning)
- PaO2 is normal. Arterial oxygen content also will be normal.
- But cells are unable to utilise oxygen resulting in high venous saturations

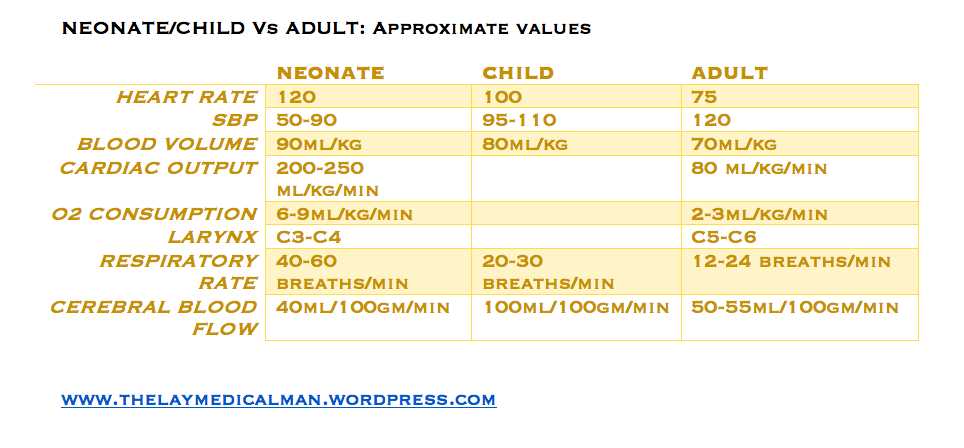
CHILD Vs ADULT; Numericals
VIVA SCENE: ADH- MECHANISM OF ACTION; ANALOGUES ; EFFECTS
MECHANISM OF ACTION:
- Anti Diuretic Hormone (ADH) stimulates V2 receptors on collecting ducts, which increases adenylate cyclase activity. This causes fusion of pre-formed water channels on the apical membrane resulting in increased permeability of the collecting ducts to water.
ADH is secreted in response to:
- Hyperosmolarity: detected by osmoreceptors in the hypothalamus, outside the blood–brain barrier. Osmoreceptors then stimulate thirst
Volume depletion (ECF) detected by low-pressure baroreceptors in great veins, atria and pulmonary vessels, and high-pressure baroreceptors in the carotid sinus and aortic arch
Angiotensin II (AGII)
- Other: pain, exercise, stress, emotion, nausea and vomiting, standing, nicotine, morphine, barbiturates, carbamazepine.
EFFECTS:
It regulates Total Body Water (TBW).
The specific renal effects of ADH on water balance include:
- Increased water permeability in cortical collecting tubule (V2 receptors)
- Increased water and urea permeability in medullary collecting tubule
- Increased retention of water
- Reduced urine volume
OTHER EFFECTS
- It stimulates thirst
- Release of factor VIII by the endothelium
- Platelet aggregation and degranulation
- Arteriolar vasoconstriction
- Glycogenolysis in the liver
- Brain neurotransmitter
- Secretion of ACTH from the anterior pituitary gland.

VASOPRESSIN RECEPTOR AGONISTS
Arginine-Vasopressin: A V receptor agonist is a potent alternative to vasoconstrictors in the treatment of fluid and catecholamine-refractory septic shock
Terlipressin is a selective V1 receptor agonist and may be more potent than arginine-vasopressin in restoring catecholamine refractory septic shock
Desmopressin (1-deamino-8-D-arginine vasopressin) is a synthetic vasopressin analog, that acts as an agonist at V1B and V2 receptors. Exhibits antidiuretic and haemostatic properties. So also used in the management of some forms of hemophilia and von Willebrand’s disease


ANTAGONISTS
Vaptans act by inhibiting vasopressin’s action on all the 3 types of receptors. e.g. Conivaptan (unselective), Tolvaptan (V2 selective). Used in the treatment of euvolemic (eg SIADH) and hypervolemic (CHF) hyponatremias, nehrogenic DI, cirrhosis, PCKD etc
VIVA SCENE: PAIN: Most important points summarised
DEFINITION:
-
Pain is ‘an unpleasant sensory and emotional experience associated with actual or potential tissue damage’. (IASP: International Association for the Study of Pain)
CLASSIFICATION:
According to chronicity
- Acute: Recent onset pain with identifiable cause
- Chronic: Pain persisting beyond the time of injury or healing without definable cause
According to nature
Nociceptive pain: Pain occurring due to stimulation of peripheral sensory nerve fibres (nociceptors) that respond to potentially harmful stimuli; further divided into
- Superficial and Deep somatic pain: Relatively well localized pain due to activation of peripheral nociceptors.
- Visceral pain (organs, viscera) – Diffuse pain that may be difficult to localize or referred to a superficial structure which is usually distant to the source of the pain
Neuropathic pain: Pain that occurs due to a primary lesion or dysfunction in the nervous system itself.
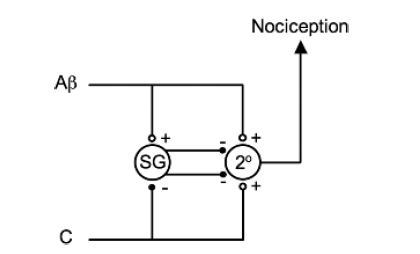
THE GATE CONTROL THEORY OF PAIN:
- Melzack and Wall theorized that the transmission of a peripheral painful stimulus to the CNS occurs via a gate at spinal cord level. This gate comprises an inhibitory interneurone in the substantia gelatinosa that may be either stimulated or inhibited by different afferent inputs.
-
The Aβ fibres are examples of afferents that stimulate inhibitory interneurones (in the substantia gelatinosa (SG)) and, therefore, prevent nociceptive transmission to the CNS. The C fibres are examples of afferents that inhibit inhibitory interneurones and, therefore, enhance nociceptive transmission. Note that both types of fibres stimulate the second-order neurone (2°) directly but it is the interneurone that modifies the transmission.
-
Laminae 2 & 3 are called the substantia gelatinosa and is the site of the ‘gate control theory’ of pain.
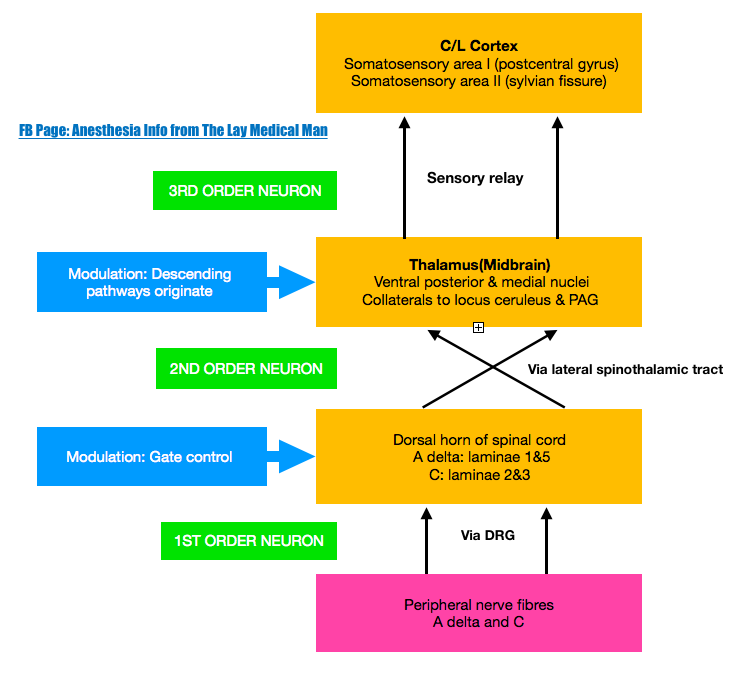
PAIN PATHWAY
-
There are three levels of neuronal involvement and the signals may be modulated at two points during their course to the cerebral cortex. Descending inhibitory pathways arise in the midbrain and pass to the dorsal horn. Multiple different neurotransmitters are involved in the pathway and include GABA,NMDA, noradrenaline and opioids.
-
Noxious stimuli –>tissue damage –>mediators–> nociceptors stimulation–> action potential –> propagated along afferent nerve fibres C & Aδ –> dorsal horn of the spinal cord –> Synaptic transmission with secondary interneurones occurs in Rexed’s laminae –> secondary interneurones decussate and travel in the anterolateral spinothalamic tracts –> through the brainstem –>to the thalamus –> tertiary afferents project to the somatosensory cortex.
-
Some spinal ascending fibres transmit impulses to the reticular-activating system, and to higher centres involved with affect, emotion and memory.
-
Descending fibres from cortex, thalamus and brainstem exert an inhibitory influence on pain transmission in the dorsal horn
-
An immediate polysynaptic withdrawal reflex occurs at the level of the spinal cord as some interneurones connect to motor neurones at many levels. This is a protective reflex.
DESCENDING INHIBITORY PATHWAYS
-
Periaqueductal grey (PAG) in the midbrain receives projections from the thalamus, hypothalamus, amygdala and cortex, and delivers projections to the nucleus raphe magnus (NRM) in the medulla, whose fibres synapse in the substantia gelatinosa of the dorsal horn. Its transmitters include endorphins and enkephalins (MOP opioid receptors) and serotonin (5HT1 and 5HT3 receptors).
-
Locus caeruleus (LC) is an important brainstem nucleus projecting descending inhibitory pathways to the dorsal horn via noradrenaline (α-adrenergic receptors).
A TRAVELOGUE: The long journey of Insulin
Insulin is produced by beta cells of islets of Langerhans.
It is produced from the pro hormone, ‘preproinsulin’ in endoplasmic reticulum. A portion of the structure is cleaved off and the remaining portion is folded with the help of C-peptide to form ‘proinsulin’
The C-peptide portion is then removed to form Insulin
This active Insulin is transported via Golgi apparatus to cytoplasmic granules for exocytosis into plasma
Insulin then binds with its receptor on Insulin sensitive cells
Insulin receptor is a tetramer consisting of 2 alpha & 2 beta units.
Insulin binds to the alpha unit on the cell membrane, while the beta unit, which spans the cell membrane activates tyrosine kinase and the second messenger system
This activates cytoplasmic vesicles containing transport molecules
The vesicles fuse with the cell membrane to incorporate the transport molecules into the cell membrane, which facilitate the transport of glucose into the cell.
MNEMO> MECHANISM OF ACTION: INSULIN Vs GLUCAGON
Insulin binding to the receptor activates an intracellular second-messenger system via tyrosine kinase.
Glucagon binding to its receptor activates a G-protein second-messenger system via adenylyl cyclase.
“Insulin is TricKy”
“Glucagon is ACcurate”
A BIOPIC ABOUT THYROXIN
Tyrosine derived from thyroglobulin is combined with iodine to produce T3 & T4 (Thyroxin)
T3 is 5 times more active than T4, though T4 is produced in larger amounts
‘ACTIVITIES OF TSH’
Increase the size & number of thyroid gland cells
Increase iodide binding
Increase the release of thyroglobulin into the colloid of the gland
Increase pinocytosis of colloid by the thyroid cells
Increase hormone production
Increase release of already produced hormone from the bound thyroglobulin into the bloodstream
In bloodstream the hormones are 99% protein bound.
Thyroxin Binding Globulin (TBG) has the greatest affinity; but Albumin has the greatest capacity for binding the hormones. Thyroxine-binding prealbumin (TBPA) also bind them
REGULATION OF HORMONAL ACTIVITY
For regulation of the hormonal levels, the negative feedback is mediated by the unbound free fraction
Stress inhibits production
Warmth decreases production
Cold increases production
Glucocorticoids, dopamine & somatostatin inhibit TSH secretion
Reference: Smith T, Pinnock C, Lin T. Fundamentals of Anaesthesia, 3rd edn. Cambridge: Cambridge University Press, 2009; p. 474 .
Iron (Fe) metabolism
We ingest dietary iron in the form of either as free Fe or as haem bound Fe
The efficiency of absorption varies & is between 5-25% only
It also depends on total body Fe stores
We cannot expel iron by metabolism: then what’s the way? (1) We depend on slow losses through cell sloughing, menstruation, bleeding etc (2) We absorb only what we want
Fe is absorbed through duodenal & jejunal mucosa
If it’s free Fe, it attaches itself to a specific receptor ( it’s expression depends on the total body Fe stores) on the apical membrane of the cell. Fe is absorbed by active transport.
If haem-bound Fe, it enters the cell by pinocytosis, and inside the cell, haem is broken down
Here it binds with apoferritin to form ferritin, which is the intracellular storage form.
When required, Fe is released into the plasma. Here it binds with the transport molecule beta-transferrin ( it’s expression is also dependent on total body Fe store)
PEAK EXPIRATORY FLOW RATE
- It’s the maximal rate of airflow during forced expiration
- Dependent on patient-effort
- Repeated 3 times and the best of the 3 is taken
- Trends in Peak Expiratory Flow helps to assess the severity status of an acute episode, response to treatment etc
- Normal values: Females: 250-500 L/min Males:450-700 L/min
- METHODS TO MEASURE:
- Wright’s Peak flow meter : Commonly used clinically and is a constant pressure variable orifice device
- Pneumotachograph : Used for research purposes and is a variable pressure constant orifice device
- From flow volume loops : They are plots of airflow at various lung volumes. Help to distinguish between obstructive & restrictive devices
Brainstem Testing for diagnosis of Brain Death
The brain stem often fails from the rostral to caudal direction and therefore it is logical to undertake testing in the same manner.
PUPILLARY REFLEXES :
The pupillary light reflex involves cranial nerves II and III and localizes to midbrain. The pupils should be nonreactive to both direct and consensual light reflex.
POINTS:
Pinpoint pupils are indicative of damage to descending sympathetic fibers as a result of damage to pons.
The size of the pupils only provides an indication of the site of brainstem involvement and is not crucial for testing brain stem death.
Clues: 2,3 pupil in MID row, some inPONdS
OCULOCEPHALIC REFLEX :
It involves cranial nerves III, VI, and VIII and interneurons within the midbrain and pons. On head movement toward right or left, the eyes remain “fixed” on a point in an intact patient. In the brain-dead patient, the eyes move with the head, hence the name “dolls eye” reflex.
POINTS:
Before performing this test the physician must rule out cervical fracture or instability.
Clues: Oh…Cee…368 dolls in MID row and PONdS
CORNEAL REFLEX :
The reflex tests the V, VII, and III cranial nerves and localizes entirely to the pons. In the intact patient, touching the cornea with a cotton swab causes eyelid closure.
The eye rotates upward, demonstrating the cranial nerve III component, known as “bell’s phenomenon.”
Corneal= sensation ; hence 5&7; plus 3 bells
OCULOVESTIBULAR REFLEX :
The oculovestibular reflex tests cranial nerves III, VI, VIII, and IV. It involves the entire pons and midbrain.
PROCEDURE :
Elevate the head 30°C. Irrigate tympanic membrane with 50-cc iced water or saline. Wait 1 min for response. Repeat test on the other side after waiting 5 min. If the oculovestibular reflex is intact using cold water as stimulus, the eyes tonically deviate toward the side of the stimulus immediately followed by a fast recoil toward the contralateral side (apparent nystagmus). In the brain stem dead patient this response is absent.
Clues: ‘broad test’ Pons & Medulla; 3,4,6, 8 COWS
GAG AND COUGH REFLEXES :
They require a functioning medulla and test cranial nerves IX and X. Both reflexes should be absent in brain stem death.
The cough reflex is easily tested by stimulation of carina by suction through the endotracheal tube. The gag reflex can be elicited by stimulating the posterior pharynx with a tongue blade.
APNEA TESTING :
This final test aims to demonstrate the failure of medullary centers to drive ventilation. Apnea test should be the last brain stem reflex to be tested.
OBJECTIVE:
Is to stimulate the medulla while avoiding hypoxia and hemodynamic compromise associated with acidosis secondary to hypercarbia.
PROCEDURE:
After ensuring preoxygenation for 10 min a blood gas is performed to confirm baseline PaCO2 and SaO2 .
With oxygen saturation greater than 95% the ventilatior is disconnected inducing apnea for a period of time to achieve ETCO2 above 6 KPa (=45 mmHg). A repeat arterial blood gases is used to confirm that the PaCO2 is at least 6 KPa and the pH is less than 7.40.
An oxygen flow rate of 2–5 L/min via an endotracheal catheter or in difficult cases CPAP may be used to maintain oxygenation till this state is attained.
Apnea is continued for a further 5 min after a PaCO2 of 6 KPa (=45 mmHg) has been achieved.
If there is no spontaneous respiratory response, a presumption of absence of respiratory activity is made.
A further blood gas can be done to confirm that the PaCO2 has risen by 0.5 KPa (=4 mmHg) from the initial 45 mmHg baseline.
Reference: Brain Death in Neurosurgical Critical Care Amit Prakash, Basil Matta , Essentials of Neurosurgical Anesthesia & Critical Care 2012
