WHAT ARE THE CAUSES OF PALPITATION
Exercise, Anxiety, Caffeine, alcohol, drugs: thyroxine, cocaine, beta 2 agonists, MI, arrthymias, hyperthyroidism, hypoglycaemia, phaeochromocytoma
MECHANISMS OF ARRHYTHMIAS
Reentry circuits, Enhanced automaticity, Triggered activity
EVALUATION AND MANAGEMENT
History and examination, ECG: 12-lead, 24-hour, ambulatory, cardiac electrophysiological study, blood investigations to rule out endocrine causes
ABC, oxygen, etc., Check electrolytes (including Mg2+), Carotid sinus massage, Adenosine – caution in asthma and if taking dipyridamole prolongs half-life
WHAT HAPPENS IN WPW SYNDROME?
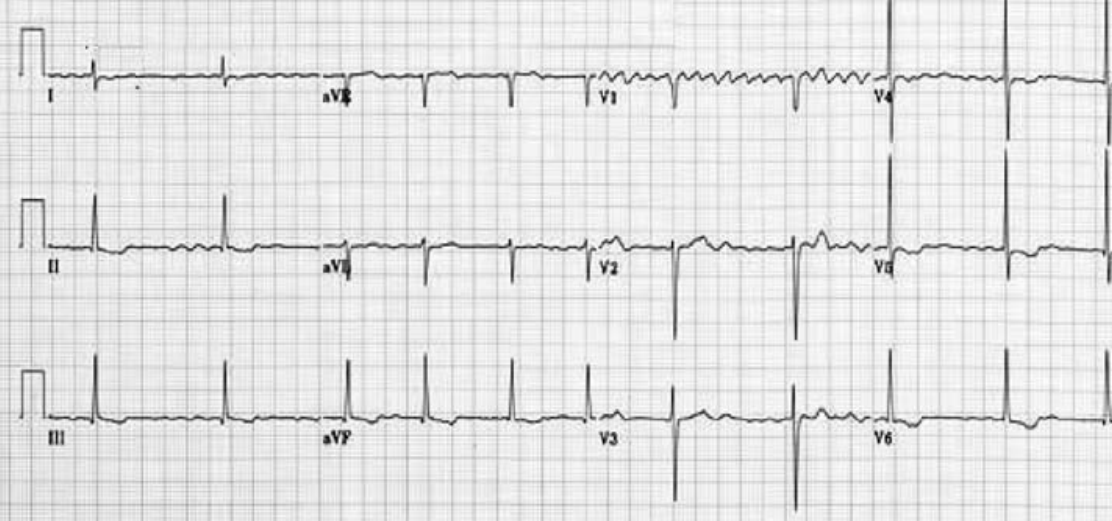
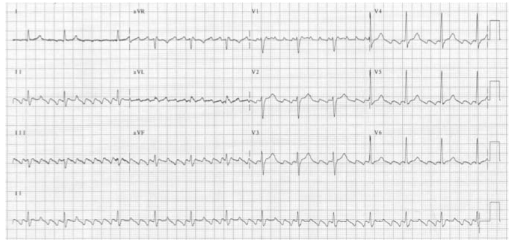
Presence of faster accessory pathway (bundle of Kent) between atrium and ventricle (accessory AV pathway) which conducts impulses faster than the normal AV node. Electrical signals traveling down this abnormal pathway may stimulate the ventricles to contract prematurely, resulting in a unique type of supraventricular tachycardia. The ECG may show sinus rhythm, normal axis, short PR interval and the presence of delta waves
DELTA WAVES
The accessory atrio-ventricular pathway conducts the atrial impulse to the ventricles much faster than the A–V node. This results in the start of ventricular depolarisation sooner than normal, hence the short P–R interval. That initial ventricular depolarisation
takes place in normal ventricular tissue (i.e. not specialised conducting tissue). The initial rate of depolarisation is therefore slower, hence the slurred, delta wave. When the rest of the impulse finally arrives the A–V node, the bundle of His and Purkinje carries out the ventricular depolarization as normal; hence, the rest of the QRS looks normal
IMPLICATIONS FOR ANESTHESIA
1.There is a tendency to paroxysmal supraventricular tachycardia in the perioperative period and there may be associated congenital cardiac abnormality.
2.Anaesthetic drugs tend to change the physiology of AV conduction.
3.If the patient is asymptomatic, then risk of perioperative arrhythmias is much less.
4.We should avoid light planes of anaesthesia and drugs that can precipitate tachycardia (like atropine, glycopyrrolate, ketamine) resulting in paroxysmal supraventricular tachycardia or atrial fibrillation
5.There are references showing disappearance of delta waves after propofol administration, making it the drug of choice for induction. For maintenance, Isoflurane and sevoflurane are preferred as they dont have effect on AV node conduction. Short acting nondepolarizing muscle relaxant would be an acceptable choice as reversal of neuromuscular blockade using neostigmine and glycopyrrolate is not required.
6. Regional anaesthesia has significant advantage over general anaesthesia as multidrug
administration, laryngoscopic stimulation, intubation, and light planes leading to sympathetic stimulation are avoided.
WHAT ARE THE COMMON ARRHYTHMIAS IN WPW?
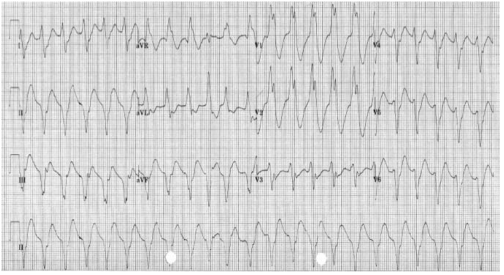
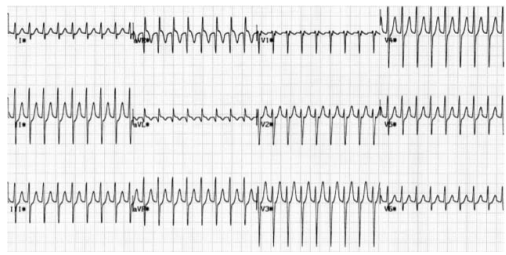
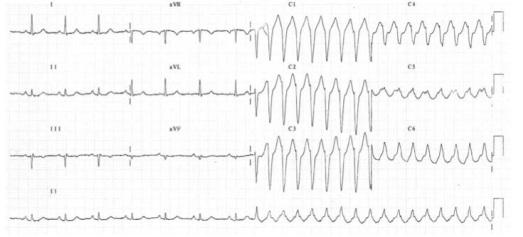
Atrial fibrillation (AF): Patients with WPW who develop atrial fibrillation are at risk of very rapid ventricular responses as the accessory pathway does not provide any ‘protective delay’ like the A-V node. This may result in heart failure or may even deteriorate into ventricular fibrillation. In AF, most conducted impulses reach the ventricles via the accessory pathway, so delta waves are seen on the ECG.
Re-entrant tachycardia: A re-entry circuit is set up. After transmitting an atrial impulse, the A–V node usually recovers before the accessory pathway. If an atrial ectopic occurs at the right time, it will transmit through the A–V node while the accessory pathway is still refractory. By the time it has done this, the accessory pathway may have recovered and the impulse will then pass through it back into the atria. As the impulses are all reaching the ventricles via the A–V node and not the accessory pathway, there are no delta waves on the ECG
INTRAOPERATIVE ARRHYTHMIAS: MANAGEMENT
1. A,B,C and Treat possible triggers of rhythm disturbance such as hypoxia, hypercarbia, acidosis, electrolyte disturbance or any cause of sympathetic stimulation.
2.Assess the degree of cardiovascular compromise. If there is significant compromise, synchronised DC cardioversion starting at 25–50 J would be the treatment of choice. If the blood pressure was stable, then the management would depend on the rhythm.
3. Pharmacological therapy:
(a) For re-entrant tachycardia, adenosine would be the first choice. Class 1a drugs such as procainamide (5–10 mg/kg) and disopyramide prolong the refractory period, decrease conduction in the accessory pathway (by blocking fast sodium channel) and may terminate both re-entrant tachycardia and AF. More conventional drugs such as amiodarone, sotalol and other beta-blockers such as esmolol may also be useful.
(b)AF: The treatment principle is to prolong the anterograde refractory period of the accessory pathway relative to the AV node. This slows the rate of impulse transmission through the accessory pathway and, thus, the ventricular rate. This is in direct contradiction to the goal of treatment of non-WPW atrial fibrillation, which is to slow the refractory period of the AV node
DRUGS THAT SHOULD BE AVOIDED
Verapamil and digoxin are contra-indicated as they both preferentially block A–V conduction thereby increasing conduction through the accessory pathway. Although verapamil could, in theory, be used to terminate a re-entrant tachycardia, its use is not advisable, because these patients may then revert to AF or flutter. A further hazard with verapamil is that a tachyarrhythmia that looks like re-entrant tachycardia may actually be VT. Adenosine would preferentially block the A–V node and therefore should not be used in AF.