Tag Archives: frca
Describing a drug in exams
- Introduction, Group
- Uses
- Chemical characteristics, Presentation
- Mechanism of action
- Routes of administration and dosing
- Pharmacokinetics: Absorption, Distribution, Metabolism, Excretion
- Pharmacodynamics: CNS/CVS/GIT/RENAL/METABOLIC/MUSCULOSKELETAL etc
- Side effects
- Special points
VIVA SCENE: THROMBOELASTOGRAPHY (TEG) AND OTHER QUESTIONS
TEG is a relatively new modality for monitoring coagulation which is very useful during management of trauma and also in the perioperative scenario..
BASIS:
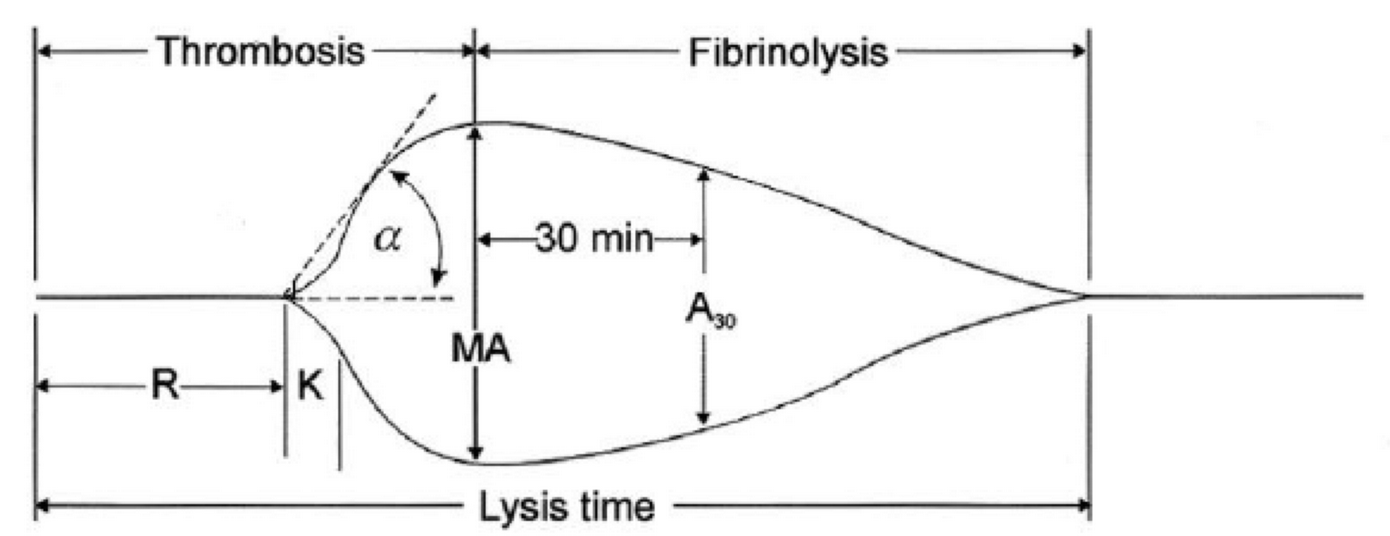
- The 2 main components of the TEG machine are a cup and a pin. Whole blood is mixed with the activating agent kaolin as well as calcium. The cup then oscillates around the pin slowly, at a rate of 6 times per minute, to mimic natural blood flow in vivo and activate the clotting cascade. As the clot forms, the torque between the cup and pin is transduced and measured, creating a curve. As the clot breaks down and torque decreases, the tracing converges to represent this.
- The different parameters of the curve are then measured to assess current coagulation status.
- Of the 4 types of TEG assays available, the most common is the rapid TEG. The use of an activator in rapid TEG standardizes the TEG test and speeds up the rate at which clotting takes place, thus making results available more quickly.
INTERPRETATION
-
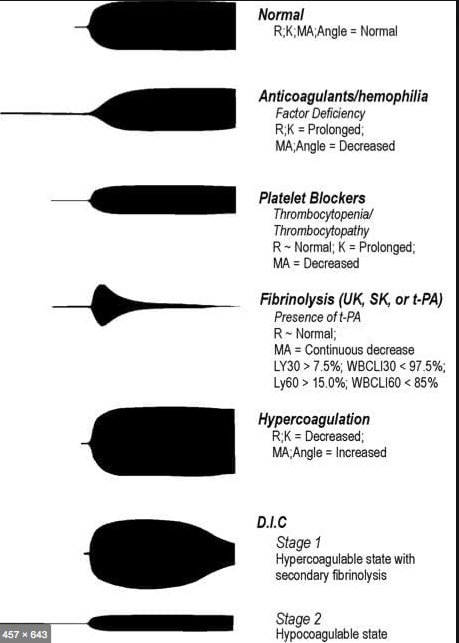
R(sec): The first measurement of note is the reaction time (R time). This is the time interval from the start of the test to the initial detection of the clot. Normal R values range between 7.5 and 15 minutes. A prolonged R time may indicate hemodilution or clotting factor deficiencies. The treatment for prolonged R time is to administer FFP as it contains all factors of the coagulation cascade, without further coagulant hemodilution. A shortening of R time (< 3 minutes) occurs in hypercoagulable states. Examples would be patients with early disseminated intravascular coagulation (DIC) or septicemia. In these situations, free thrombin is released into the circulating blood, triggering the clotting mechanisms but the patient later begins to bleed because of exhaustion of clotting factors.
-
K (sec) and Angle α (°): The clot strength is measured by these 2 variables in TEG. The K value measures the interval between the R time and the time when the clot reaches 20 mm. Normal K values range between 3 and 6 minutes. Prolongation of the K value with normal platelet count indicates inadequate amounts of fibrinogen to form fibrin. The treatment for prolonged K value is therefore to administer fibrinogen/cryoprecipitate. The α angle measures a line tangent to the slope of the curve during clot formation.The alpha angle represents the thrombin burst and conversion of fibrinogen to fibrin. Normal α value is between 45° and 55°. A longer K value causes a shallow or more acute angle (<45°), while a shorter K value causes a steeper α angle (>45 °). An angle α <45° suggests a less vigorous association of fibrin with platelets. In this case, treatment begins much higher on the coagulation cascade, with the replacement of both fibrinogen and factor VIII. Thus, these patients can be treated with the administration of cryoprecipitate. Shortening of the K-value indicates a very quick formation of clot, potentially due to hypercoagulability or inappropriate consumption of coagulation factors. A shortened K value also corresponds to a steeper α (>45°). The treatment for shortened K and steeper α is anticoagulation therapy
- MA (mm): Maximum amplitude is a measurement of maximum clot strength and provides information on both fibrinogen and platelet function. As the clot develops and increases in tensile strength due to platelet activation and binding to fibrin, the tracing increases it’s MA or appears to widen. Normal values are between 50–60 mm. 80% of the MA is derived from platelet function whereas the remaining 20% is derived from fibrin. A low MA value is indicative of low clot strength, which can be caused by decreased fibrinogen levels, low platelet counts, or decreased platelet function. (i) Paired with a prolongation of K value, this could be a sign of the need for cryoprecipitate. (ii) Administration of platelets may be avoided when a low platelet count is combined with a normal MA value (=platelet function is normal) (iii) Treatment with platelets may be indicated for patients with a low MA value (=low platelet function) and normal platelet count. (iv) High MA will occur in the setting of hyperactivity of platelets, and MA above 75 mm indicates a prothrombotic state. In this case, treating with an anticoagulant would be helpful
- Shear Elastic Modulus Strength, G value or G: is a measure of clot strength or clot firmness, and is calculated based on the amplitude value (A) until the maximum amplitude (MA) is reached. It is the single most important value of the entire assay because it represents the overall function or effectiveness of the clot. Normal G values are between 5.3 and 12.4 dynes/cm2. A G value >10 dynes/cm2 indicates increased risk of thrombosis. Treatment for high G is accomplished by the use of platelet inhibitors such as Clopidogrel or Aspirin. Aspirin is usually not preferred because it inhibits platelet adherence rather than platelet aggregation. A G <5 dynes/cm2 places a patient at increased risk of hemorrhage
- As time progresses during the TEG assay, the tracing will remain at maximal amplitude for a period of time, after which clot lysis begins. Normally, lysis continues for a period of up to 15 minutes. A computerized algorithm automatically estimates the percentage of lysis occurring over time. This is called the Estimated Percentage of Lysis or EPL. After 30 minutes, EPL becomes EPL30 or succinctly LY30 (i.e. percentage of lysis at 30 minutes). Both the EPL and LY30 are measurements of excessive fibrinolysis since they measure the percentage decrease in amplitude after MA. An EPL between 7.5 and 15%, when accompanied by a very high G, reflects a hyperfibrinolytic and hypercoagulable state typical of patients with early DIC. A very high EPL or LY30 (>20%) may indicate the need for antifibrinolytic therapy, such as the use of transexamic acid or aminocaproic acid. LY30 is also useful for patients undergoing thrombolytic drug therapy. This can be observed by rapid curve convergence.
Ref: Thromboelastography: Clinical Application, Interpretation, and Transfusion Management, Shawn Collins et al AANA Journal Course, 2016
HOW DO WE TEST CLOTTING?
- By doing tests like aPTT, PT & INR, Platelet Count, ACT, Bleeding Time, fibrinogen and factor levels, TEG etc
- The aPTT and INR use different reagents to measure the time to form a clot in vitro after platelet-poor plasma from blood collected in a calcium chelating tube, is recalcified
- The aPTT is prolonged with the deficiency of factors of the intrinsic pathway: Fs 8,9,11,12. Also the factors involved in the common pathway (Fs 1,2,10)e.g. Heparin therapy, DIC, liver disease
- The INR is prolonged especially with deficiency of F 7; but also with deficiency of Fs 1,2,5,10 e.g. warfarin therapy, vitamin K deficiency, DIC, liver disease
- N.B Warfarin inhibits the gamma carboxylation of vitamin K dependent factors 2,7,9,10
- BLEEDING TIME :Duke’s method: Sterilize the finger tip using rectified spirit and allow to dry. Make a sufficiently deep prick using a sterile lancet, so that blood comes out freely without squeezing. Note the time (start the stop-watch) when bleeding starts. Mop the blood by touching the finger tip with a filter paper. This is repeated every 15 seconds, each time using a fresh portion of the filter paper, till bleeding stops. Note the time (stop the stop-watch). Normal value is upto 4 minutes.
- CLOTTING TIME: Capillary tube method: (Wright’s method). Under sterile precautions make a sufficiently deep prick in the finger tip. Note the time when bleeding starts (start the stop watch). Touch the blood drop at the finger tip using one end of the capillary tube kept tilted downwards. The tube gets easily filled by capillary action. After about two minutes start snapping off small lengths of the tube, at intervals of 15 seconds, each time noting whether the fibrin thread is formed between the snapped ends. Note the time (stop the stop watch) when the fibrin thread is first seen. Clotting time is the interval between the moment when bleeding starts and the moment when the fibrin thread is first seen.
Normal value is 3 to 10 minutes.
- Bleeding time depends on the integrity of platelets and vessel walls, whereas clotting time depends on the availability of coagulation factors
VIVA SCENE: CARBON MONOXIDE (CO) POISONING
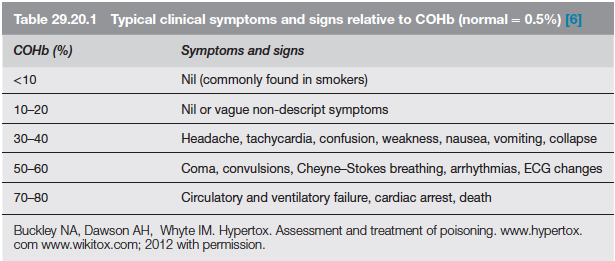
AETIOLOGY: Carbon monoxide is produced by incomplete combustion and is found in car exhaust, faulty heaters, fires and in industrial settings. Carboxyhaemoglobin (COHb) concentrations in cigarette smokers range as high as 10%.
MECHANISM: Binds to Hb with 210 times affinity than O2: so reduce the O2 carrying capacity of blood. Also disrupts oxidative metabolism, binds to myoglobin and cytochrome oxidases, causes lipid peroxidation. Final result is tissue hypoxia. Severity depends on the duration of exposure, CO levels and patients pre-event health status: pre-existing cerebral disease, cardiac failure, hypovolemia and anemia increase toxicity
DIFFERENTIAL DIAGNOSIS: Cyanide poisoning ( suspected when CNS effects are out of proportion with COHb concentrations and if there is a marked lactic acidosis)
EVALUATION & MANAGEMENT:
- ABC approach
- Secure the airway; if GCS<8, consider intubation
- Stabilize respiration: consider mechanical ventilation or CPAP
- Get intravenous access
- Send samples for estimation of Hb(?anemia), electrolytes (dyselectrolytemias worsen the cardiac toxicity), COHb levels (to confirm diagnosis; useless in prognosis), blood sugar, ABG and cardiac enzymes. Take an ECG.
- Metabolic acidosis due to lactate give a clue to the extend of ischemia. Net effect of metabolic acidosis may be beneficial on O2 delivery; but treated if pH<7
- Patient discouraged from activity
- 100% O2 reduces the half life of COHb from 4 hours (in ambient air) to 40 minutes. 4 to 6 h of 100% normobaric oxygen will remove over 90% of the carbon monoxide. Oxygen toxicity is unlikely with less than 24 h treatment
- When immediately available, hyperbaric oxygen (HBO) should be considered with serious CO poisoning. Oxygen at 2–3 atmospheres will further reduce the half-life of COHb to about 20 min but, more importantly, it causes very rapid reversal of tissue hypoxia due to oxygenation of tissue from oxygen dissolved in the plasma. Some clinicians implement it based on the presence of any of the following: history of loss of consciousness, abnormal neuropsychiatric testing or neurological signs, pregnancy. COMPLICATIONS OF HBO: decompression sickness, rupture of tympanic membranes, damaged sinuses, oxygen toxicity
- CO PRODUCTION WITH SODALIME USE: Occurs when inhalational agents with CHF2 moiety such as desflurane, enflurane, and isoflurane are used with desiccated soda lime granules that was left unused for a long time. Can be significant in smokers especially when very low flows are used. Factors increasing the production of CO include° Type of inhaled anaesthetic agent (magnitude of CO production from greatest to least is desflurane > enflurane > isoflurane > sevoflurane)° High absorbent dryness ° Type of absorbent (at a given water content, baralyme produces more CO than soda lime)° Increased temperature° Higher anaesthetic concentration
Broad Complex Tachycardias
A broad complex tachycardia has a QRS complex greater than 0.12 seconds. They are usually ventricular in origin, but can also be supraventricular with aberrant conduction. Other possible causes for broad complex tachycardias include atrial fibrillation with ventricular pre-excitation, i.e. patients with Wolff–Parkinson–White (WPW) syndrome, or torsades de pointes (polymorphic VT).
Broad complex tachycardia is therefore due to SVT with aberrancy or Ventricular Tachycardia (VT), and differentiating between the two can be challenging. However, there are a few pathognomonic ECG features that diagnose VT
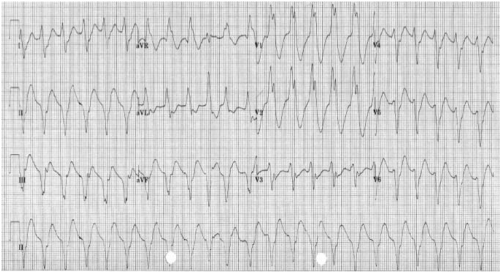
1.Atrio-ventricular (AV) dissociation. There is a higher ventricular rate than atrial rate (more QRS complexes than P-waves). This can only occur if the ventricular rate is autonomous and no longer under control of the SA node.
2.Capture beats: There is an isolated narrow complex amongst a train of broad complexes. This represents a normally conducted P-wave via the AV node and an intact His-Purkinje system indicating there is no underlying bundle branch block. Therefore, the train of broad complexes are ventricular in origin (i.e. VT).
3.Fusion beats: A normally conducted P-wave may fuse with a simultaneous ventricular beat causing a complex halfway between the appearance of a normal QRS and a broad complex.
4.VT is more likely in patients with a prior history of MI.
5.VT complexes are usually very broad (> 160 ms) due to a very abnormal path taken by the depolarisation wave from the VT focus.
6.The time from R-wave onset to the nadir of the S-wave is prolonged (> 100 ms) in VT, again representing an abnormal activation path through the ventricle.
7.Extreme left axis deviation and positive aVR are more common in VT, as the ventricles are depolarised in the opposite direction to normal conduction.
8.Failure to respond to iv adenosine
9.The absence of typical RBBB or LBBB patterns suggests VT. For example, an RSR pattern in V1 with a taller first R-wave suggests VT (in RBBB the first R-wave is caused by septal depolarisation and is therefore smaller than the second R-wave, which is caused by depolarisation of the RV).
SVT with aberrancy is more likely if previous ECGs demonstrate an accessory pathway or a bundle branch block with identical morphology to the broad complex tachycardia. When in doubt, treat as VT
TORSADES DE POINTES
Is a specific variant of ventricular tachycardia (VT). It has a classic undulating pattern with variation in the size of QRS complex. It is caused by a prolonged QT interval and can precipitate VF and sudden death
QT PROLONGATION: CAUSES
Tricyclic antidepressants, flecainide and quinidine; Hypocalcemia; Acute myocarditis
VENTRICULAR TACHYCARDIA(VT) AND VENTRICULAR FIBRILLATION (VF)
VT is a broad complex tachycardia, defined as a run of at least three consecutive ventricular ectopic beats, at a rate of >120 bpm. Can arise from a single or multiple foci or from a reentry circuit. There may be capture or fusion beats, where a normally conducted beat will join an ectopic beat travelling in the opposite direction
CAUSES: Acute MI, degeneration of other arrhythmias, electrolyte abnormalities etc
VF describes an ECG which is random and chaotic with no identifiable QRS complexes that is incompatible with life and need immediate provision of ACLS with prompt delivery of DC shock. Others: Amiodarone, Lidocaine, beta blockers, Implantable cardioverter defibrillators
MANAGEMENT
For VT treat with amiodarone 300 mg IV followed by 900 mg over 24 hours. If the arrhythmia is known to be supraventricular, treat as a narrow complex tachycardia.
An irregular broad complex tachycardia is most likely to be atrial fibrillation with bundle branch block, and should be treated as narrow complex atrial fibrillation
In a stable patient who is known to have WPW, the use of amiodarone is probably safe. Adenosine, digoxin, verapamil and diltiazem must be avoided, as these drugs block the AV node and will cause a relative increase in pre-excitation
Torsades de pointes is treated by stopping all drugs known to prolong the QT interval and correcting electrolyte abnormalities. Magnesium sulphate (2 g IV over 10 minutes) should also be given. Such patients may require ventricular pacing. If the patient’s condition deteriorates proceed to synchronised electrical cardioversion or, if the patient is pulseless, commence the ALS algorithm
Ventricular bigeminy
Ventricular bigeminy is associated with endotracheal intubation (a sympathoadrenal response). Given time the bigeminy will disappear, but if it does not intravenous
lidocaine (50–100 mg) may be helpful
Narrow Complex Tachycardias
Narrow complex tachyarrhythmias have a QRS duration <0.12 seconds. They arise above the bundle of His.
NARROW COMPLEX TACHYCARDIA
As narrow complex tachycardias involve ventricular activation through the normal His-Purkinje system, they must originate within the atria and are therefore often referred to as supraventricular tachycardia (SVT). There are five common types of SVT. They are: Atrial tachycardia, Atrial fibrillation, Atrial flutter, Atrioventricular nodal
re-entry tachycardia, Atrioventricular re-entry tachycardia. When faced with an ECG of narrow complex tachycardia, (i) we should examine the P-wave and (ii) check the QRS regularity
SINUS ARRHYTHMIA/TACHYCARDIA/BRADYCARDIA (from SA Node)
ATRIAL FIBRILLATION
There is completely disorganised atrial activity, with P-waves replaced by an irregular baseline due to fibrillation waves, and QRS complexes occur in an irregularly irregular fashion (Please the post on AF)
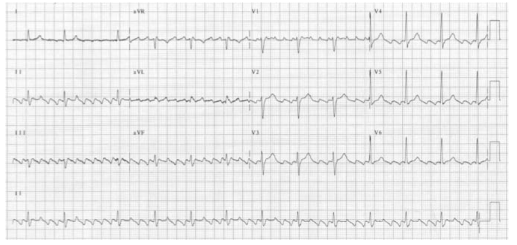
ATRIAL FLUTTER
There is a self-perpetuating wave of atrial depolarisation usually circulating within the right atrium, causing regular, saw-toothed flutter waves at 300 bpm and QRS complexes every second, third, or fourth flutter wave. We can see classical sawtooth flutter waves.Drug control of the ventricular rate is not often successful.
ATRIAL TACHYCARDIA
There is an abnormal atrial focus driving the ventricular rate. This rhythm can be difficult to distinguish from sinus tachycardia, but P-wave morphology and axis is usually abnormal. If the atrial focus is close to the AV node, a junctional tachycardia may occur and P-waves may be absent.
In case of Atrial tachycardia with AV block after halting glycoside therapy (and ensuring normokalaemia), lidocaine 1 mg kg−1 IV is the drug
of choice. Alternatively DC cardioversion or atrial
pacing may be effective.
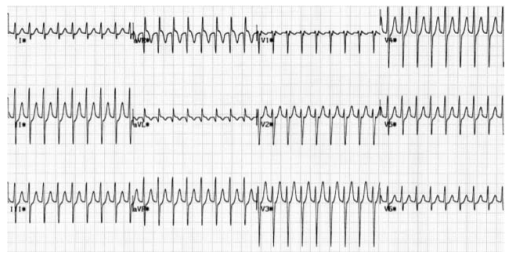
ATRIO VENTRICULAR NODAL REENTRY TACHYCARDIA (AVNRT)
This is the commonest type of paroxysmal supraventricular tachycardia (PSVT). It is often seen in people without any heart disease, and is usually benign. There is a rapid reentry circuit within the AV node resulting in simultaneous atrial and ventricular depolarisation. The P-wave is usually buried within the QRS or ST-segment. There will be fast regular narrow complex tachycardia, and P-waves can be seen buried in the terminal portion of the QRS complex which may easily be mistaken for a second, small R-wave. The very close proximity of the QRS and P-waves implies near simultaneous depolarisation of atria and ventricles.
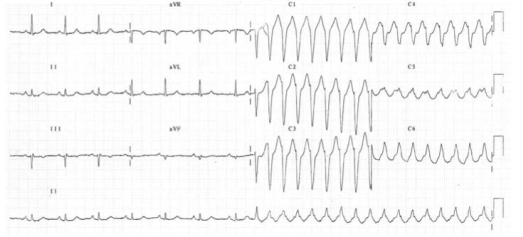
ATRIO VENTRICULAR REENTRY TACHYCARDIA (AVRT)
This occurs in patients with WPW, and is usually benign unless there is coexisting structural heart disease. There is an accessory pathway bridging the atria and ventricles allowing antegrade conduction down the AV node (causing a narrow QRS) and retrograde conduction back to the atria via the accessory pathway. Since the depolarisation wave takes time to complete this circuit, the P-wave occurs after the QRS complex and is often buried within the T-wave. AVRT can occur with antegrade conduction to the ventricles via the accessory pathway, but this will result in ventricular depolarisation via an abnormal route and consequently a broad QRS. In sinus rhythm, antegrade conduction via the accessory pathway produces a short PR interval (as the normal delay in the AV node is avoided) and the abnormal activation of the ventricles produces a slurred upstroke
in the QRS called a delta wave. The QRS complex is said to be pre-excited and can be associated with repolarisation abnormalities. There are seven sinus beats followed by a ventricular ectopic beat that conducts to the atria retrogradely through the atrioventricular node and then returns to the ventricles via the accessory pathway. This cycle repeats and triggers a broad complex tachycardia. (Please see post on ‘WPW Syndrome’ also).
An unstable patient presenting with a regular narrow complex tachycardia should be treated with electrical cardioversion. If this is not immediately available, adenosine should be given as a first-line treatment. A stable patient presenting with a regular narrow complex tachycardia should initially be treated by vagal
manoeuvres such as carotid sinus massage or the Valsalva manoeuvre, as these will terminate up to a quarter of episodes of PSVT. Carotid sinus massage should be avoided
in the elderly, especially if a carotid bruit is present, as it may dislodge an atheromatous plaque and cause a stroke
Management
A stable patient presenting with a regular narrow complex tachycardia should initially be treated by vagal manoeuvres such as carotid sinus massage or the Valsalva manoeuvre, as these will terminate most episodes of PSVT. Carotid sinus massage should be avoided in the elderly, especially if a carotid bruit is present, as it may dislodge an atheromatous plaque and cause a stroke. If the tachycardia persists and is not atrial flutter, 6 mg of adenosine should be given as an IV bolus, followed by a 12 mg bolus if no response. A further 12 mg bolus of adenosine may be given if the tachycardia persists. Vagal manoeuvres or adenosine will terminate almost all AVNRTs or AVRTs within seconds, and therefore failure to convert suggests an atrial tachycardia such as atrial flutter. If adenosine is contraindicated, or fails to terminate a narrow complex tachycardia, without first demonstrating it as atrial flutter, give a calcium-channel blocker, e.g. verapamil 2.5–5 mg IV over two minutes. Atrial flutter should be treated by rate control with a beta-blocker.
An irregular narrow complex tachycardia is most likely to be atrial fibrillation (AF) with an uncontrolled ventricular response, but may also be atrial flutter with variable block. If the patient is unstable, synchronised electrical cardioversion should be used to treat the arrhythmia
ATRIAL FIBRILLATION (AF) AND THE ANESTHESIOLOGIST
Atrial fibrillation(AF) is a supra-ventricular arrhythmia characterized by the complete absence of co-ordinated atrial contractions. There will not be any discernable p-waves.
The ventricular response rate depends on the conduction of the AV node.
WHAT IS THE DIFFERENCE BETWEEN ATRIAL FIBRILLATION AND ATRIAL FLUTTER
Flutter is a more organised and regular form of atrial activity and classically with an atrial rate of 300 bpm. ‘Saw toothed’ flutter waves are present on the ECG. The ventricular response depends on conduction through the AV node. The classic ECG has 2:1 block, hence a ventricular rate of 150 bpm
CAUSES OF AF IN THE PERIOPERATIVE SETTING
Electrolyte abnormalities especially low potassium or magnesium
Withdrawal of beta blockers
Following cardiac surgery.
ASD or mitral valve disease
Ischaemic heart disease
Thyrotoxicosis
Excess caffeine or alcohol (acute or chronic)
Pulmonary embolism
Pneumonia
Pericarditis
In the context of major vascular surgery, systemic inflammation,hypovolemia and a heightened adrenergic state are likely to play a major role.
WHAT IS LONE AF?
‘Lone AF’ is AF in the absence of any demonstrable medical cause, but this is not usually diagnosed in the peri-operative period. So beta blockers will be efficacious in this setting.
WHAT ARE THE PROBLEMS AF CAN POSE?
Loss of the atrial ‘kick’ as it contracts and empties into the LV can reduce the CO by 10%–20% with a normal ventricle (reduced by 40%–50% in those with a ‘stiff’ ventricle as in
diastolic dysfunction, aortic stenosis etc). The disorganised contractions of the atria cause stasis of blood and the risk of thromboembolism. There is a 3%–7% annual risk of
thromboembolic CVA
AF- EVALUATION
*History *Assessment of volume status and electrolytes *ECG: This will also help to exclude acute ischaemia. *The pulse will be irregularly irregular. *No ‘a wave’ in the jugular venous pulsation as this is caused by sinus atrial contraction. *Chaotic atrial activity can be seen on echocardiography.
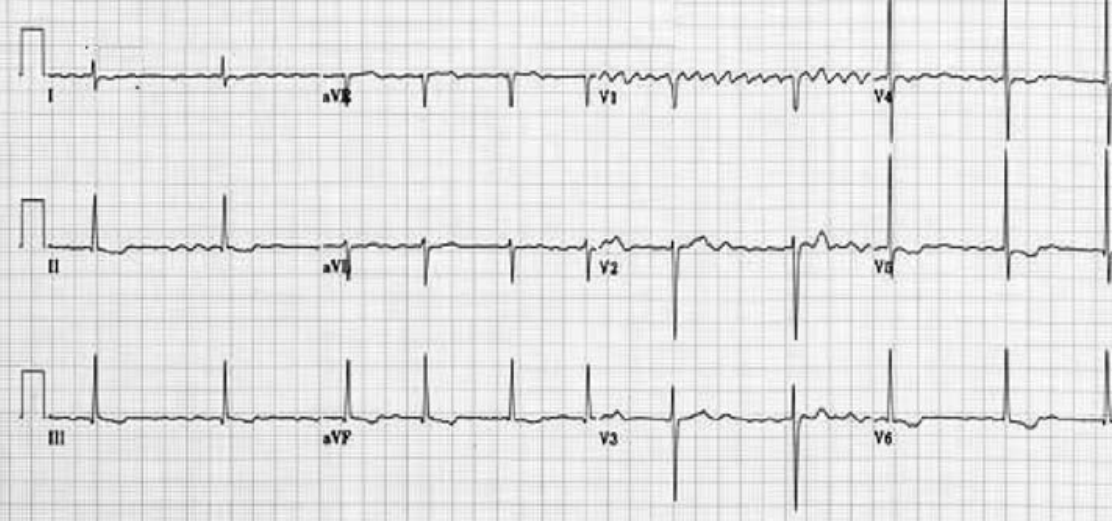
READ THIS ECG

MNEMONIC ‘RIAS QRST’ (Rate & Rhythm, Intervals, Axis, QRS & ST segment T wave)
The rate is 78 bpm; the rhythm is irregularly irregular. There are flutter waves seen in the V1 rhythm strip. The axis is normal (There is borderline LVH by voltage criteria). There are no Q waves and the QRS width is normal. There is evidence of infero-lateral ischaemia shown by the inverted and biphasic T waves in this territory (II, III, aVF and V3−V6).
MANAGEMENT OF AF
Assess for cardiovascular compromise and resuscitate simultaneously if needed. Oxygen should be administered, continuous ECG monitoring instituted and IV access secured.
If the patient is unstable, synchronised electrical cardioversion should be used to treat the arrhythmia.
In a stable patient, treatment options include: Rate control by drug therapy. Drugs used to control the heart rate include beta-blockers, digoxin, magnesium, the non-dihydropyridine calcium channel blockers (verapamil or diltiazem) or a combination of these.
Rhythm control by amiodarone to encourage cardioversion: Amiodarone is given as a 300 mg IV bolus, followed by 900 mg IV over 24 hours.
Rhythm control by electrical cardioversion: This is more likely to restore sinus rhythm than chemical cardioversion.
Treatment to prevent complications. Patients who are in AF are at risk of atrial thrombus formation and should be anticoagulated
Patients who have pre excitation syndromes with an accessory conduction pathway between the atria and ventricles (such as in the Wolff–Parkinson–White syndrome) should not be given AV node blocking drugs if they develop an SVT. This will promote the atrial impulses to travel directly to the ventricle at up to 300 bpm via the accessory pathway. The drugs of choice are amiodarone, flecainide or procainamide.
BEFORE PROCEEDING WITH DC CARDIOVERSION FOR AF, WHAT ALL THINGS SHOULD BE CONSIDERED?
- Cardioversion should only be attempted without anticoagulation if the duration of the AF is less than 48 hours. If the duration is unknown or longer than this, 3–4 weeks of anticoagulation (INR 2–3) is required to reduce the incidence of clot embolisation. If there is a contra-indication to anticoagulation, or if the cardioversion is deemed necessary more urgently, then an echocardiogram is needed to exclude thrombus in the atrium and atrial appendage
- When did the AF start (history of palpitations or recording on monitor): is it acute or chronic?
- What is the likelihood of an atrial thrombus which could be embolised by
cardioversion? - What is the ventricular rate now? – may need pacing after cardioversion if
the rate is below 60 bpm - Has there been an ischaemic episode?
ANESTHESIA FOR CARDIOVERSION
This should be done in a critical care or operating room area with the usual preparation, equipment and assistance needed for any routine anaesthetic. Someone independent should be present to perform the defibrillation, preferably with a hands-free device. Elective cardioversion has been done under conscious sedation without any adverse effects, but the usual technique is to use a sleep dose of propofol following pre oxygenation. One can use a facemask or maintain the airway with an LMA. If there is any serious doubt about cardiovascular performance or reserve, an arterial line should be given consideration, but this is a short procedure and the cardiac output should improve with the restoration of sinus rhythm. If the patient has a pacemaker in situ or an implantable cardiac defibrillator, we should place the paddles as far away as possible from the device and preferably in the anterior–posterior position.
IF THE PATIENT DOES NOT GET CARDIOVERTED, WHAT SHOULD YOU DO?
Try a period of 4–6 weeks of medical therapy and anticoagulation. If the patient is still in AF, then a further trial of DCC is reasonable. If a second DCC is unsuccessful, then rate control is the next step to improve symptoms and reduce ventricular failure.
EDAIC & FRCA TIPS FROM VARIOUS SOURCES 2005-2019 (Mainly for PART 2)
The examination aims to assess a candidate’s knowledge of:
•The basic sciences
•Clinical anaesthesia (including obstetric anaesthesia & analgesia)
•Resuscitation and emergency medicine
•Specialist anaesthesia (e.g. neuro-, cardiac, thoracic, paediatric)
•Intensive care
•Management of chronic pain
•Current literature
BASIC SCIENCES
- anatomy, biochemistry, physiology, applied physiological measurement, pharmacology, physics and principles of measurement, STATISTICS
CLINICAL ANESTHESIOLOGY - preop assessment, GA & RA , postop care NEONATAL RESUSCITATION RESUSCITATION & EMERGENCY MEDICINE
- Basic Life Support and Advanced Life Support. Pre-hospital care. Immediate care of patients with medical or surgical emergencies, including trauma
INTENSIVE CARE AS FOLLOWS:
- Both acute surgical and medical conditions.
- Use of assessment and prognostic scoring systems.
- Parenteral and enteral nutrition.
- Biochemical disturbances such as acid base imbalance, diabetic keto-acidosis, hyperosmolar syndrome and acute poisoning.
- Renal failure including dialysis.
- Acute neurosurgical/neurological conditions.
- Patients with multiple injury, burns and/or multi-organ failure.
- Principles of ethical decision-making.
MANAGEMENT OF CHRONIC PAIN AS FOLLOWS:
- The physiology of pain.
- management of pain.
- The concept of multidisciplinary care.
- Terminal care
- CURRENT LITERATURE
Candidates will be expected to be conversant with major topics appearing in current medical literature related to anaesthesia, pain relief and intensive care.
Whilst national and linguistic differences are recognised, some knowledge is expected on topics of international importance (e.g. new agents) even if they are not in current use in all countries.
It must he stressed that the foregoing is NOT intended either as an examination syllabus or as a comprehensive list of topics covered by the examination. It is however, a guide, which it is hoped will prove useful to candidates preparing for the diploma examination
GUIDANCE FOR CANDIDATES SITTING THE PART II EDAIC (2019)
The Part II EDAIC is an oral examination. Not all candidates are familiar with this type of examination and the following notes are intended to provide some guidance with regard both to preparation and to performance on the day.
The examination of each candidate is held in a single day during which there are four 25-minute oral examinations – (or vivas, as they are known) – two in the morning and two in the afternoon. In each of these, the candidate is examined by a pair of examiners, thereby meeting eight examiners in all. As far as possible, candidates are not examined by examiners from their own training hospital. The two morning vivas concentrate on applied basic sciences and the afternoon vivas relate to clinical topics.
Usually, but not invariably, each pair of examiners comprise one whose mother tongue is that of the language in which the candidate has chosen to be examined and the other who has a good working knowledge of the language. It is accepted that candidates may not be using their mother tongue and some allowance for linguistic difficulties is made.
In the vivas, the examiners use “Guided Questions” (GQ’s) which have been set in advance by the examination committee. Each GQ opens with a brief scenario. Ten minutes before the viva, the scenario is handed to the candidate. It is written in his/her chosen language. This gives the candidate time to collect his/her thoughts and prepare to answer questions on the topic presented. These opening questions are then followed by questions on the other topics listed in the examiner’s GQ. The first examiner asks questions for the first 12½ minutes after which a bell rings and the second examiner takes over.
Note that, whereas the Part I EDAIC basic science MCQ’s are designed to test factual recall of relevant basic science knowledge, the Part II basic science vivas are designed to test that the candidate understands the relevance of basic science knowledge applied to the practice of anaesthesia and critical care. Thus pharmacology, physiology, anatomy and relevant clinical measurement and instrumentation will always be tested. Similarly, the Part I EDAIC clinical MCQ papers are mainly concerned with testing the candidate’s factual clinical knowledge whereas the Part II clinical vivas are concerned with testing the understanding and application of that knowledge
CURRENT FORMAT OF THE EDAIC PART II EXAMINATION (2019)
The GQ’s with which the examiners are supplied list topics to be discussed with indications as to the detail required. The general format of the exam is as set out below.
MORNING
Viva 1 (Applied Basic Science)
This will start with the scenario the candidate was given 10 minutes before the start of the viva and will include applied cardiovascular and/or respiratory physiology. It will then move on to applied anatomy and physiology of other organs and systems.
Viva 2 (Applied Basic Science)
This will start with the scenario the candidate was given 10 minutes before the start of the viva and will include applied pharmacology. It will then move on to clinical measurement, applied pharmacology/physiology combined.
AFTERNOON
Viva 3 (Clinical – Critical care subject)
This will start with questions on the intensive care or emergency medicine scenario the candidate was given 10 minutes before the start of the viva. Questions on the scenario will be followed by topics such as clinical management, X-ray/CT/MRI/Ultrasound images interpretation, anaesthetic specialties and general questions.
Viva 4 (Clinical – Management of an anaesthetic problem)
This will start with questions on the anaesthetic problem scenario the candidate was given 10 minutes before the start of the viva. Questions on the scenario will be followed by questions on an internal medicine topic – possibly related to the scenario. There will also be questions on local or regional anaesthesia and some general questions.
MARKING
For each of the 20 topics of the day, each examiner can award one of three marks, which indicate respectively:
Pass ‘2’. The candidate’s performance will be deemed: fluent, able to apply knowledge, confident on core topics, thorough and able to demonstrate appropriate depth, able to correct own errors,
Borderline ‘1’. The candidate’s performance will be deemed: showing factual knowledge only (book learning with no explanation, showing poor or incomplete understanding, superficial – particularly with core topics, erratic/unstructured/disorganized, illogical but with no dangerous clinical decisions.
Fail ‘0’. The candidate’s performance will be deemed: not answering question asked despite prompting or silence, showing evidence of severe lack of topic understanding, offering multiple answers for examiner to pick, having a dangerous clinical approach
All the marks of the eight examiners (two examiners for each four sessions) will be added up to make the final score of the candidate.
To be successful, the candidate needs to obtain:
1. a score of at least 25 out of 40 in the morning sessions (Viva 1 + Viva 2)
2. a score of at least 25 out of 40 in the afternoon sessions (Viva 3 + Viva 4)
3. an overall score of at least 60 out of 80
Thus, it can be seen that, at the meeting of examiners at the end of the day, in the majority of cases there need be no further discussion of individual candidates. However, if a candidate has obtained a final score of 59, the examiners concerned would be asked to justify the mark.
Some reasons for candidates failing include:
• Inability to apply knowledge and/or basic science to clinical situations
• Inability to organise and express thoughts clearly
• Unsound judgement in decision-making and problem-solving
• Lack of knowledge and/or factual recall
In essence the examiners ask themselves the following questions:
a) Does the candidate have a good foundation of knowledge? Can the candidate apply that knowledge and understand its relevance to the practice of anaesthesia and intensive care?
b) How does the candidate approach a problem? Is the approach logical and well thought out?
c) Have alternative options been explored and understood? Is the candidate dangerous?
The Part ll examination may only be taken after the candidate has completed his/her training for specialist accreditation in their respective country. A wide general knowledge in anaesthesia, intensive care and subjects allied to anaesthesia is therefore expected.
Background Reading
Which books shall I read? How much detail is required? These are common questions. There is no simple answer particularly since the EDAIC is an international exam, and the examiners and candidates come from different backgrounds. A basis for reading is the standard text book(s) of anaesthesia favoured in the candidate’s country. Familiarity with current topics from international and national journals is also be required. Access to journals may vary in different departments but the Internet now provides a wealth of new opportunities. In addition, a recommended reading is also at your disposal.
THE FOLLOWING ADDITIONAL POINTS MAY BE OF ASSISTANCE
Applied Basic Science Vivas
Physiology
It is obvious that the physiology of the cardiovascular and respiratory systems will be examined in some detail. A good knowledge of neuro, renal and hepatic physiology as applied to anaesthesia and intensive care will also be expected. Other areas relevant to anaesthesia will also be covered but great detail is not expected.
Pharmacology
The principles of pharmacokinetics and pharmacodynamics will be examined in some detail. An intimate knowledge of the pharmacology and toxicology of drugs used in anaesthesia is expected as well as many of the drugs in common use in intensive care. An informed anaesthetist who reads journals must have some understanding of research protocols and the relevance of statistical methods employed, in order to judge the value of articles.
Applied Anatomy
It is expected that anaesthetists will know the essential anatomy of areas into which they may insert needles cannulae and endotracheal and endo-bronchial tubes. Applied anatomy of the heart and lung is also examined.
Physics and Clinical Measurement
Anaesthetists monitor and measure numerous clinical parameters and take action on the information displayed. It is expected therefore that they should understand the principle of action, limitations, accuracy, and sources of error in these monitors. Some of the basic physics of gases and vapours, and principles of electrical safety are essential knowledge for the informed anaesthetist. The principle of action and causes of failure in anaesthetic machines and ventilators is also essential knowledge.
Clinical Anaesthesia & Intensive Care Vivas
Clinical Anaesthesia
As candidates will have completed their training to the standard required for specialist registration they should have experience in all types of anaesthesia and intensive care. These vivas will include questions on both general, regional and special anaesthetic techniques as applied to neuro-, cardiac and paediatric surgery, obstetric anaesthesia and the management of acute and chronic pain.
The examiners do not have direct experience of how the candidate would deal with an anaesthetic problem. They therefore have to make a judgement based upon the candidate’s performance in the oral exam. The examiner cannot assume the candidate would have carried out a procedure or checked a clinical or electronic monitor. The candidate must mention it.
Clinical scenario
An example of the clinical scenario given in advance to a candidate would be as follows: A 67-year-old man weighing 100kg, 1.67m in height is scheduled for an elective repair of a 10cm abdominal aortic aneurysm. He had myocardial infarction 6 months previously and has been a non-insulin dependent diabetic for over 10 years. Discuss your anaesthetic management of this case.
The initial discussion on this sort of opening scenario will reveal much about the candidate’s approach to the problem and an awareness of the potential dangers. Remember that the anaesthetic management starts in the ward!
Definition of problems: Clearly, the primary problem is the presenting aneurysm and its repair. What will it involve?
Secondly the patient is obese and has, as yet unquantified, cardiovascular problems and diabetes.
This would lead to a full medical history with emphasis on the above with appropriate examination and investigation of potential complications. The anaesthetic management would involve choice of technique, appropriate monitoring, management of complications and post-operative pain relief.
A candidate who presents a logical well structured answer, explaining the reasons behind the proposed course of action, is more likely to find that the examiner says very little and does not have to interject continually. It cannot be emphasised enough that practice in presentation is essential and candidates should practice this skill with their trainers or fellow trainees. This is even more important for candidates not using their mother tongue
This topic alone, could take up more than the allotted time and so examiners may suddenly curtail discussion on a given subject and move on to something else. This is a necessary part of the examination process and does not indicate displeasure with the answers given.
Candidates should appreciate that the intention of the examiners is to enter into a dialogue with them regarding whatever topic is under discussion. The intention is not simply to find the candidate’s areas of ignorance although, inevitably, these may become apparent – if they exist. Bearing this in mind, the candidate should try to discuss the topic knowledgeably and should not be afraid to say when the topic is completely outside his/her experience. The EDAIC being an international exam and not a collection of national exams, means inevitably, that a wide range of views will be held both by the candidates and examiners.
It is assumed that candidates have been trained in standard mainstream anaesthetic techniques. They would be wise therefore to base their answers on methods with which they are familiar and would be normal in their institution, rather than straying into unfamiliar territory in the mistaken belief that this might be the answer the examiners require. Examiners will sometimes query an answer to see whether the candidate is confident in their answer or can be swayed from their course of action. There will often be no right or wrong answer to a question and examiners will accept an answer or opinion that is based on sound evidence and justifies the proposed course of action
SYSTEMATIC REVIEW OF
Images
Candidates are expected to have a systematic and logical approach to reading Images and should be able to describe their system to the examiner. A typical system would be:
For example, for X-rays:
Markings: Look at writing on the film: name/age of the patient and projection of the radiograph.
Film Quality: Penetration, rotation & inspiration (on a chest film).
Review Areas: Lungs, diaphragm, pleura, upper abdomen, heart & mediastinum, bones of thoracic cage & soft tissues.
Artifacts: Note the presence of any equipment placed in the chest by anaesthetists or surgeons!
Recognition of Critical Incidents and taking prompt and appropriate action
One common cause for failure in the exam is a haphazard approach to dealing with critical situations that are posed and not following Advanced Life Support protocols. Airway, Breathing and Circulation should be the foundation of all resuscitation.
Diagrams & Graphs
Use of diagrams, graphs and other material to present answers. Pencils and paper are provided at all times during the Part II vivas. Candidates can use them to advantage in making presentations and explaining points. A typical scenario given in advance in the applied basic science exam might be: Discuss the factors that influence carriage of oxygen in the blood. A diagram of the various oxy-haemoglobin dissociation curves with some relevant values would create a good impression at the commencement of the exam and help the candidate settle into a structured answer. In pharmacology, the value of diagrams and graphs in explaining the principles of pharmacodynamics or pharmacokinetics is obvious .
N.B. For EDAIC 1
1-2 Questions from statistics will come in Part 1 Paper A
Statistics: Basic principles of data handling, probability theory, population distribution and the application of both parametric and non-parametric tests of significance.
GENERAL ADVICE ON PRACTISING FOR VIVAS (Basically for FRCA; but may be useful for EDAIC too)
We found the following techniques extremely valuable in the run-up to the vivas:
1.Group revision
2.Frequent practice
3.Practise categorising
4.Card system
- Group revision: It is extremely useful to team up with some friends or colleagues regularly in the weeks before the viva and practise talking about anaesthetic topics. Practising with friends has several advantages: Seeing your friends on a regular basis will help keep you sane. This is better than locking yourself in a small room with a pile of books and trying to learn the coagulation cascade for the fifth time since qualification! Your morale will remain in better shape than if you were revising on your own because you will be able to encourage each other. You will also be more aware of the progress you are making. As a group, you can pool your resources in terms of reference books and previous questions. During the working day, one of you may have had a practice viva with a consultant who asked an awkward question or a common question asked in a different way. You can then discuss with your friends how they would have answered it. Different people revise in different ways and, consequently, will have their own way of talking about a subject. This means that others in the group will benefit from listening to the practice viva. They may have a particular piece of knowledge that really helps an answer gel together or they may use a particular turn-of-phrase that succinctly deals with a potential minefield. You can practise phrasing your answers in a particular way in the knowledge that, if it all falls apart halfway through, it won’t matter and you can have another go. This is less easy to do in front of consultants who might write your reference! By being ‘the examiner’, you will gain insight into the pitfalls of the viva process. You can usually see someone digging a hole for themselves a mile off!
- Frequent practice: Repetition of clinical scenarios. During your revision, you will find the same clinical situations coming up time and time again (as in the exam). Over the years, anaesthetic techniques may change but new techniques are all aimed at trying to solve particular clinical problems, for example, the fibre-optic scope to help with the difficult airway or new drugs that provide more cardiovascular stability. However, the problems remain the same! Patients will still present with difficult airways, ischaemic heart disease, COAD, obesity, hypertension, etc. The more you practise, the more often you will find yourself repeating the problems each of these scenarios presents and thus the more confident and slick you will become at delivering the salient points. There are obviously a few exceptions, e.g. MRI scanners and laser surgery, where the advancement of technology has presented new challenges to the anaesthetist. These situations are in the minority and as long as you are aware of them and the associated anaesthetic problems, you should be well-equipped to deal with questions on them in the exam.
- The clinical scenarios break down into a few categories:
- Medical conditions that have anaesthetic implications, e.g. Aortic stenosis, Diabetes, Hyperthyroidism.
- Surgical procedures that have anaesthetic implications, e.g. Oesophagectomy, CABG, Pneumonectomy.
- Anaesthetic emergencies/difficult situations, e.g. Anaphylaxis, Malignant hyperthermia, Failed intubation. Paediatric cases. These represent a limited range of cases the examiners are likely to ask you about, e.g. Upper airway obstruction, Pyloric stenosis, Bleeding tonsil.
- Having repeatedly practised these clinical scenarios, you will soon realise that the problems of anaesthetising an obese patient with diabetes, ischaemic heart disease, porphyria and myasthenia for an abdominal aortic aneurysm repair (!) can be broken down into the problems that the respective conditions present to the anaesthetist, plus the problems of the specific operation. You may then approach what seems to be a nightmare question with a degree of confidence and structure.
- Phrasing: It cannot be over-emphasized that frequent practice will improve your viva technique. As already mentioned, some topics crop up again and again in different situations, such as part of a long case or even a complete short case (e.g. obesity, anaesthesia for the elderly or the difficult airway). With regular practice, you will soon develop your own ‘patter’ to help you deal with these common clinical scenarios. These can then be adopted at opportune moments to buy yourself easy marks whilst actually giving you time to gather your thoughts
- Practise categorising: Putting order to your answers demonstrates to the examiners that you conduct your clinical practice in a systematic and safe way. If you do not mention the most important points first (e.g. airway problems in a patient presenting with a goitre), then this may suggest to the examiners that you are disorganized. An ‘ABC’ (order of priority) approach to many of the questions may be helpful. For example, in obese patients, managing the airway has a higher priority than difficulty with cannulation. It is often a good idea to use your opening sentence to tell the examiners how you are going to categorize your answer.
Example 1:
‘Tell me about the anaesthetic implications of rheumatoid arthritis’.
‘Patients with rheumatoid arthritis may have a difficult airway and secondary respiratory and cardiovascular pathology. They are frequently anaemic, taking immunosuppressant drugs and the severe joint pathology leads to problems with positioning’.
Example 2:
‘What are the important considerations when anaesthetising a patient for a
pneumonectomy’?
‘These may be divided into three broad areas: the pre-operative assessment of fitness for pneumonectomy and optimisation, the conduct of anaesthesia with particular reference to one-lung anaesthesia, positioning, intra-operative monitoring and fluid balance and finally post operative care’.
- Card system: We formatted postcards to summarise the main problems associated with different anaesthetic situations. These proved to be a good starting point for viva practice and a quick source of reference. They also encouraged us to deliver the first few points in a punchy manner.
For example:
‘What problems do you anticipate with anaesthetising a patient with Down’s syndrome’?
‘These patients present the following problems for the anaesthetist. They may have a difficult airway, an unstable neck, cardiac abnormalities, mental retardation, epilepsy and a high incidence of hepatitis B infection’.
VIVA TECHNIQUE
- 1.Think first: Don’t panic. If you are unlucky enough to be asked a question about an obscure subject such as lithium therapy (as two of us were in our science viva), remember the examiners have only just seen the questions as well. It may also be of some comfort to know that there will be at least ten other candidates being asked the same question at the same time. Keep things simple at first and think about how you are going to structure your answer. Categorising your answer may allow you to deliver more information about the topic than you thought you knew. Conversely, do not dwell on what you do not know, e.g. the pH and dose!
Example: ‘Tell me about lithium’
Think . . . ‘What is it used for’?
Say . . . ‘Lithium is a drug used in the treatment of mania and the prophylaxis of manic depression’.
Think . . . ‘What is the presentation and dose? . . . I don’t know the dose’.
Say . . . ‘It is presented in tablet form’.
Think . . . ‘What is its mode of action? . . . I have no idea but I know it is an antipsychotic’!
Say . . . ‘Its main action is as an antipsychotic’.
Think . . . ‘Why are they asking me this question? What is the relevance to anaesthetic practice’?
Say . . . ‘It has a narrow therapeutic range and therefore toxicity must be looked for. Side effects may include nausea, vomiting, convulsions, arrhythmias and diabetes insipidus with hypernatraemia’.
A similar approach can be used for the clinical viva.
- The opening sentence : This will set the tone of the viva. If the first words to come from your mouth are poorly structured, ill thought-out or just plain rubbish, then you are likely to annoy the examiners and will face an uphill struggle. If, on the other hand, your first sentence is coherent, succinct and structured, then you will be half-way there. With a bit of luck, the examiners will sit back, breathe a sigh of relief (because it has been a very long day for them) and allow you to demonstrate your obvious knowledge of the subject in hand!
For example:
‘What are the problems associated with anaesthesia for thyroid disease’?
‘Anaesthesia for patients with thyroid disease has implications in the pre-, intra- and post-operative periods’.
You are then able to expand in a logical way from here.
‘Pre-operatively, assessment of the airway and control of the functional activity of the gland is essential . . . ’
- Categorise or die!
Remember this lends structure to your answer and gives the examiners the impression you are about to talk about the subject with authority. If you categorise your answer well enough, they may actually stop you and move onto something else.
- Opening question
You will be asked to summarise the case so prepare your opening sentence
beforehand.
For example:
You may be asked to summarise the scenario of a 75-year-old man with chronic obstructive pulmonary disease who is scheduled to undergo an elective cardio-oesophagectomy the following day.
‘Would you like to summarise the case’?
One possible answer may begin:
‘This is an elderly gentleman with complex medical problems who is scheduled for a cardio oesophagectomy. He has evidence of chronic obstructive pulmonary disease, ischaemic heart disease and diabetes. There will be substantial strain on his cardio-respiratory system. This operation is a major procedure that involves considerable fluid shifts, a potential for large blood loss and requires careful attention to analgesia. These are the main issues that I would concentrate on in my pre-operative assessment’.
Even though a cardio-oesophagectomy involves other considerations (e.g. double-lumen tube / one-lung ventilation) it can be seen that this opening sentence could be adapted to suit other clinical scenarios such as:
Pneumonectomy
Laparotomy
CABG / valve replacement
Cystectomy
Open prostatectomy
- Analyse all the investigations
You will be asked for your opinion on the ECG, chest X-ray, blood results, etc., so make sure you have decided on the abnormalities and the most likely causes for them in the 10 minutes you have to view the data. Try to make your answers punchy and authoritative.
For example, ‘The ECG shows sinus rhythm with a rate of 80 and an old inferior infarct’ is better than going through the ECG in a painstaking ‘The rate is . . . the rhythm is . . . the axis is . . . ’
Don’t waste valuable time waffling on about the normal-looking bones on a chest X-ray if there is a barn-door left lower lobe collapse. This does not necessarily imply you are not thorough, providing you demonstrate that you have looked for and excluded other abnormalities.
- Anaesthetic technique
You will usually be asked how you would anaesthetise the patient in the long case. There will often not be a right or wrong answer, but you should try to decide on your technique and be able to justify it. The examiners may only be looking for the principles of anaesthesia for a particular condition such as aortic stenosis, although this is probably more likely in the short cases.
For example:
‘You are asked to provide an anaesthetic for a 77-year-old lady who needs a hemi-arthroplasty for a fractured neck of femur. She had a myocardial infarction 3 months ago and has evidence of heart failure’.
You should be able to summarise the principles involved and choose an anaesthetic technique appropriate to the problems presented. You could, for example, give this patient a general anaesthetic with invasive monitoring , you could use TIVA with remifentanil or a neuroaxial block.
All of these techniques could be justified, but to simply say that you would use propofol, fentanyl and a laryngeal mask without saying why, may be asking for trouble!
In some circumstances it may be the options for management rather than a specific technique that is required. You may find it appropriate to list the options for analgesia in a patient having a pneumonectomy, for example, and then say why you would use one technique over the others.
You should try to address the anaesthetic technique for the long case BEFORE you face the examiners. You will not look very credible if you have had 10 minutes to decide on this and have not reached some kind of conclusion. Overall, most candidates felt that the examiners were pleasant and generally helpful. If you are getting sidetracked they will probably give you a hint so you do not waste time talking about something for which there are no allocated marks. If they do give you a hint, take it!
Good luck.
CONTEXT SENSITIVE HALF TIME [CSHT]
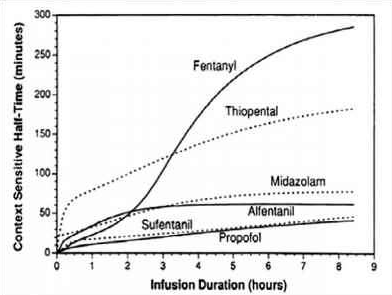
-
Context sensitive half-time is deined as the time for the plasma concentration to fall to half of the value at the time of stopping an infusion
-
The half time will usually alter in the setting of varying durations of drug infusion
- The higher the ratio of distribution clearance to clearance due to elimination, the greater the range for context-sensitive half-time
- The longest possible context-sensitive half-time is seen when the infusion has reached steady state, when there is no transfer between compartments and input rate is the same as elimination rate
- Draw and label the axes; draw the curve for the drug with the shortest CSHT first before plotting the others
- REMIFENTANIL: Here the elimination always dominates distribution and so there is very little variation in CSHT with time and so it is context insensitive. Draw a straight line starting from the origin and becoming near horizontal after the CSHT reaches 5 min. This demonstrates that the half time is not dependent on the length of infusion as clearance by plasma esterases is so rapid. For remifentanil the
longest possible CSHT is only 8 minutes
- PROPOFOL: For propofol the clearance due to elimination is similar to that for distribution into the second compartment, so plasma concentration falls rapidly after a propofol infusion mainly due to rapid elimination with a smaller contribution from distribution. Propofol is not context insensitive as its CSHT continues to rise; however it remains short even after prolonged infusions. Starting at the origin, draw a smooth curve rising steadily towards a CSHT of around 40 min after 8 h of infusion.
- ALFENTANIL: The curve rises from the origin until reaching a CSHT of 50 min
at around 2 h of infusion. Thereafter the curve becomes horizontal. This shows that alfentanil is also context insensitive for infusion durations of 2 h or longer
- THIOPENTONE SODIUM: The curve begins at the origin but rises more steeply than the others so that the CSHT is 50 min after only 30 min infusion duration. The
curve should be drawn like a slightly slurred build-up exponential reaching a CSHT of 150 min after 8 h of infusion. As the CSHT continues to rise, thiopental does not become context insensitive
-
FENTANYL: The most complex curve begins at the origin and is sigmoid in shape. It should cross the alfentanil line at 2 h duration and rise to a CSHT of 250 min after 6 h of infusion. Again, as the CSHT continues to rise, fentanyl does not become context insensitive.
-
The maximum possible CSHT for propofol is about 20 minutes, compared with 300 minutes for fentanyl
-
It is important to realize that the CSHT does not predict the time to patient awakening but simply the time until the plasma concentration of a drug has fallen by half. The patient may need the plasma concentration to fall by 75% in order to awaken, and the time taken for this or any other percentage fall to occur is known as a decrement time.
-
Decrement time: The time taken for the plasma concentration of a drug to fall to the specified percentage of its former value after the cessation of an infusion designed to maintain a steady plasma concentration (time). The CSHT is, therefore, a form of decrement time when the ‘ specified percentage’ is 50%.
-
Although the CSHT for propofol has a maximum value of about 20 minutes, during long, stimulating surgery infusion rates will have been high and the plasma concentration when wake-up is required may be very much less than half the plasma concentration at the end of the infusion. Thus time to awakening using propofol alone may be much longer than the CSHT. This is why the TCI pumps display a decrement time rather than a CSHT.
-
When using propofol infusions, the decrement time is commonly quoted as the time taken to reach a plasma level of 1.2 μ g.ml−1 , as this is the level at which wake up is thought likely to occur in the absence of any other sedative agents.
-
It must be remembered that after one CSHT, the next period of time required for plasma concentration to halve again is likely to be much longer. This relects the increasing importance of the slower redistribution and metabolism phases that predominate after re-distribution has taken place. This explains the emphasis on half-time rather than halflife: half-lives are constant whereas half-times are not!
VIVA SCENE: COMPATIBILITY IN BLOOD TRANSFUSIONS: RBC Vs FFP Vs PLATELETS AND OTHER QUESTIONS
COMPATIBILITY: RBC TRANSFUSION
In red cell transfusion, there must be ABO and RhD compatibility between the donor’s red cells and the recipient’s plasma.
All healthy normal adults of group A, group B and group O have ANTIBODIES IN THEIR PLASMA against the red cell types (antigens) that they have not inherited
Among the ABO blood groups:
Group A individuals have antibody to group B
Group B individuals have antibody to group A
Group O individuals have antibody to group A and group B
Group AB individuals do not have antibody to group A or B. So,
1 Group O individuals can receive blood from group O donors only ( as the antibodies against A or B in their plasma will react with any A or B antigens which enter the circulation)
2 Group A individuals can receive blood from group A and O donors
3 Group B individuals can receive blood from group B and O donors
4 Group AB individuals can receive blood from AB donors, and also from group A, B and O donors ( as their plasma don’t have any antibodies against any antigens)
RhD RED CELL ANTIGENS AND ANTIBODIES
Is the second most important group system. Out of the existing C,D and E antigens, D is the most antigenic one. Anti D antibodies are not normally found in the blood of Rh negative individuals; instead they develop it only when itcomes into contact with Rh positive blood during child birth or inappropriate transfusion. In case of subsequent transfusins or pregnancies with Rh positive blood- this can cause rapid destruction of RhD positive red cells (Hemolytic disease of the newborn[HDN] in subsequent pregnancies; to prevent this sensitization we should give Rhesus imunoglobulin= Anti-D prophylaxis- to the Rh negative mother who gave birth to an Rh positive baby). The fetal red cells are haemolysed, causing severe anaemia. HDN due to ABO incompatibility is usually less severe than Rh incompatibility.). FFP does not need to be Rh-compatible. Anti-D prophylaxis is not necessary in Rh D-negative recipients of Rh D-positive FFP.
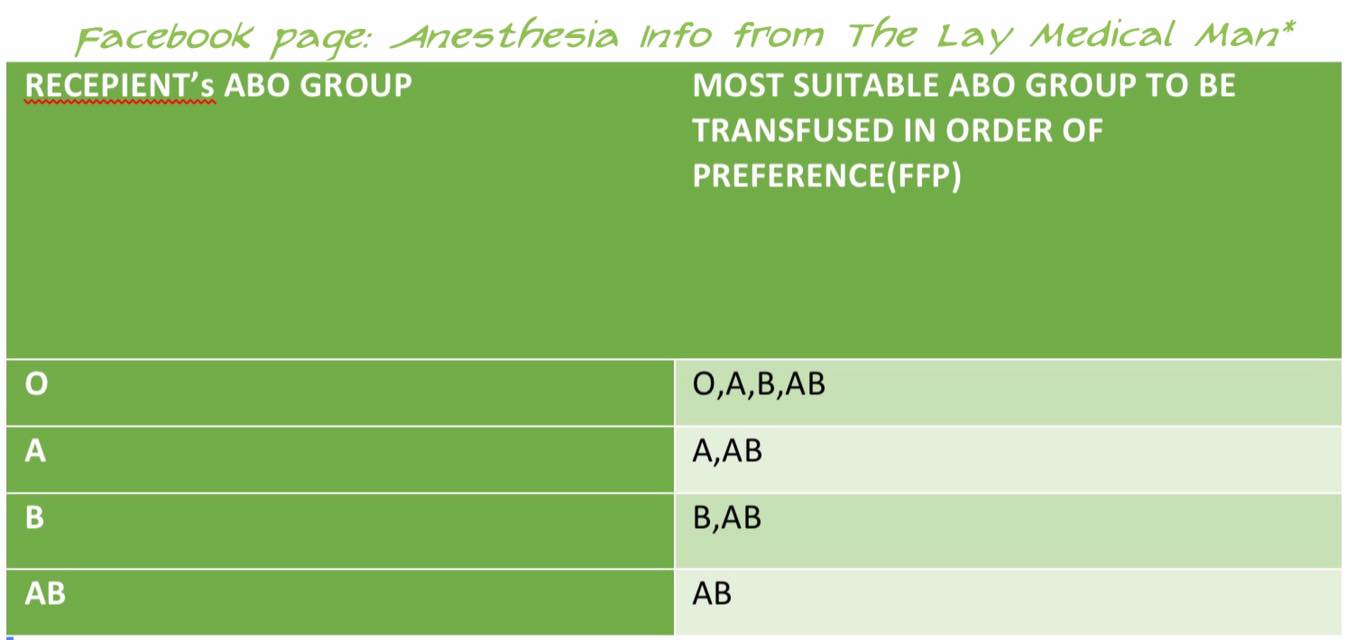
PLASMA TRANSFUSION: COMPATIBILITY
In plasma transfusion, group AB plasma can be given to a patient of any ABO group because it contains neither anti-A nor anti-B antibody.
1 Group AB plasma (no antibodies) can be given to any ABO group patients
2 Group A plasma (anti-B) can be given to group O and A patients
3 Group B plasma (anti-A) can be given to group O and B patients
4 Group O plasma (anti-A + anti-B) can be given to group O patients only
FFP does not need to be Rh-compatible (However, the unit will still be labelled as Rh +ve or Rh −ve) ; anti-D prophylaxis is not necessary in Rh D-negative recipients of Rh D-positive FFP
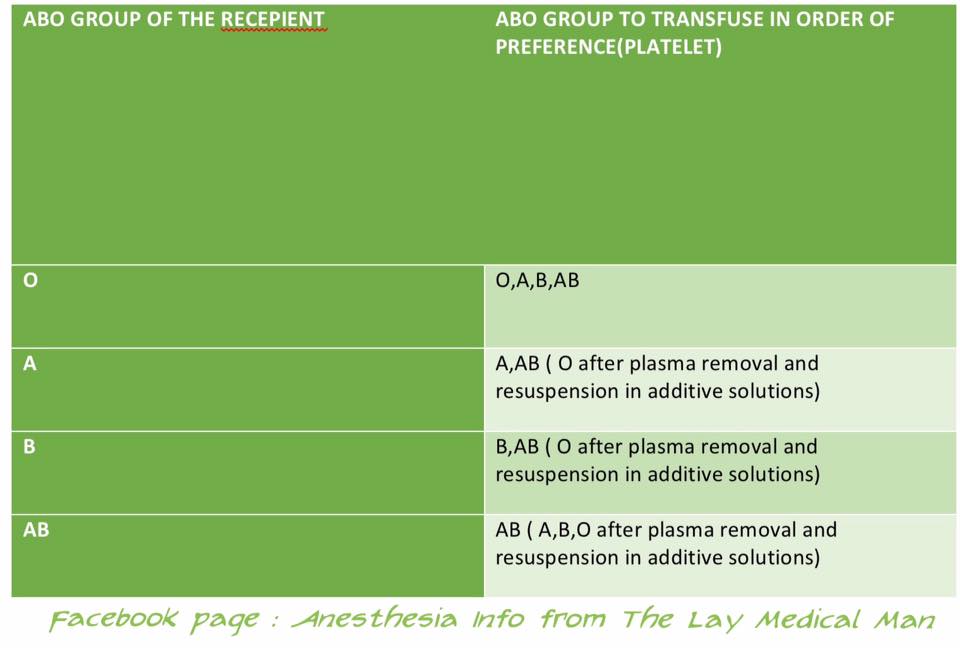
PLATELET TRANSFUSION: COMPATIBILITY
The Platelet Concentrates( PCs ) transfused must be ABO-identical, or at least ABO-compatible, in order to give a good yield ( Ideally, ABO identical units should be used but, in an emergency, ABO non-identical units can be used, although the improvement seen in platelet count post-transfusion may be less.)
Group O PC can be used for patients with blood groups A, B, and AB ONLY IF, they are resuspended in additive/preservative solutions, or if negative for high titre anti-A/A,B
ABO-incompatible PCs have reduced efficacy and, preferably, should not be used
Rh-negative patients, in particular women of childbearing age, should receive, if possible, RhD-negative PC
In the case of a transfusion of a RhD-positive PC to a RhD-negative women of childbearing age, 250 IU (50 μg) of anti-D immunoglobulin should be administered, a dose able to cover the transfusion of five therapeutic doses of PC in 6 weeks
ACUTE EMERGENCY : COMPATIBILITY
During an acute emergency, the blood bank may send group O (and possibly RhD negative) blood, especially if there is any risk of errors in patient identification. This may be the safest way to avoid a serious mismatched transfusion, in such situations.
HOW A GROUP AND SAVE IS PERFORMED? (P’s = Patient’s)
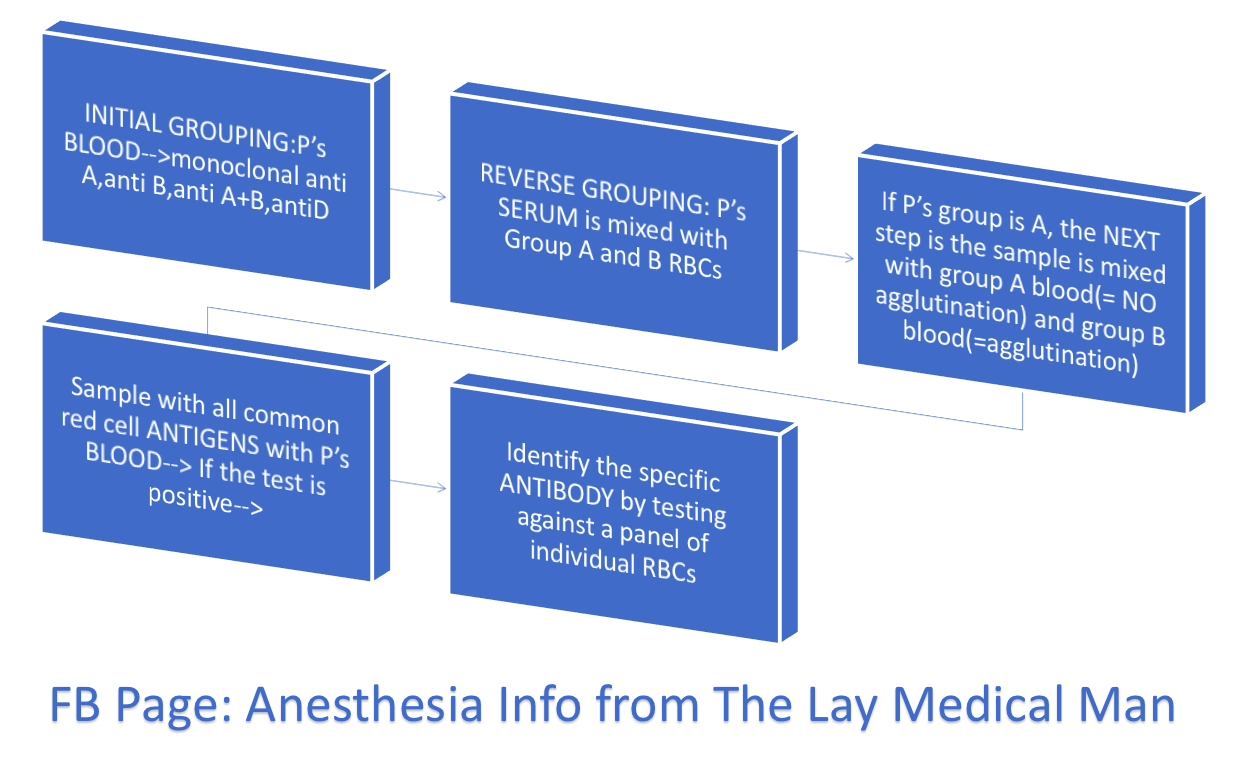
#BloodTransfusion , #ABO , #BloodGroup , #TransfusionMedicine , #Anaesthesia , #Anesthesia , #Bloodbank
Reference: The Clinical Use of Blood, Handbook, WHO,
Recommendations for the transfusion of plasma and platelets Giancarlo Liumbruno, Francesco Bennardello, […], and as Italian Society of Transfusion Medicine and Immunohaematology (SIMTI) Working Party
AAGBI GUIDELINES 2016
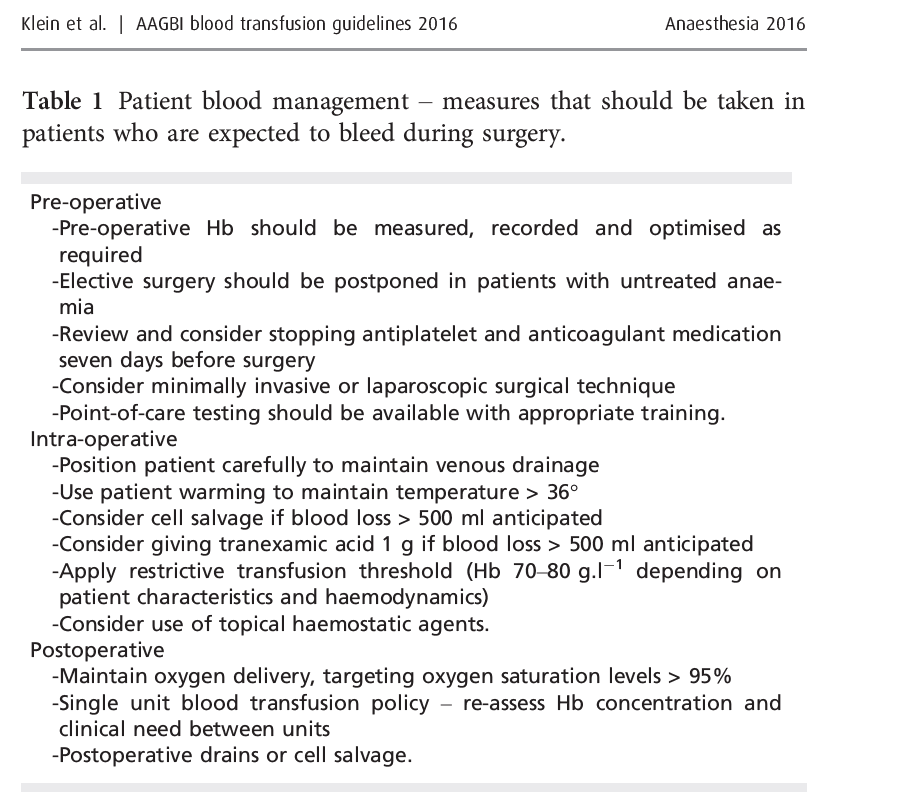
HOW WILL YOU TRANSFUSE THE BLOOD?
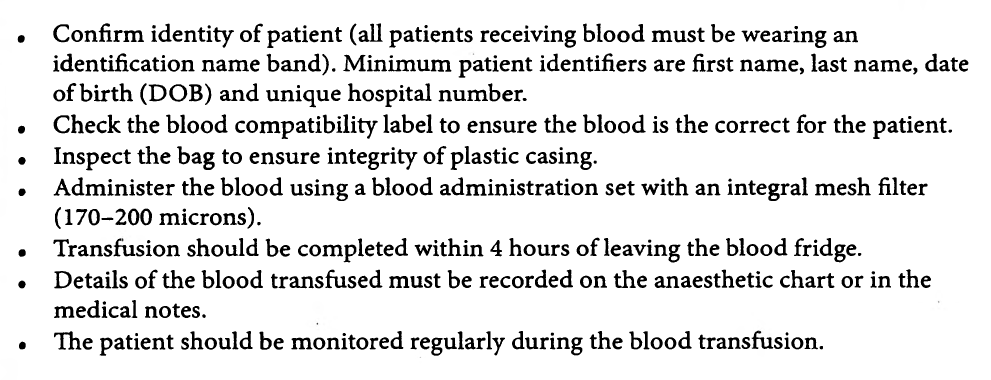
Also preoperatively the need for transfusion must have been explained and written informed consent should have been taken
A general Hb threshold of 7.0 g/dl should apply as a guide for red cell transfusion. 8.0 g/dl for patients with IHD
ALSO NOTE:
• A transfusion of 10 ml/kg of RBC should increase Hb by approximately 2.0 g/dl-
• Cryoprecipitate should be given in a dose of 5–10 ml.kg-1
• Platelets should be given in a dose of 10–20 ml.kg-1.
• Fresh frozen plasma may be given in doses of 10–15 ml.kg-1.
Tranexamic acid can be used in children: a loading dose of 15 mg.kg-1 followed by infusion 2 mg.kg-1.h-1 should be used in trauma
