STRESS ULCER RISK FACTORS
Stress ulceration in intensive care patients is relatively common (approaching 90% by day 3 with no prophylaxis), although the incidence of clinically important GI bleeding is less than 2%. There are six major risk factors: 1.Respiratory failure requiring ventilation for >48 hours 2.Coagulopathy 3.Sepsis 4.Hypotension 5.Hepatic failure 6.Renal failure
STRESS ULCER PATHOPHYSIOLOGY
- Impaired mucosal blood flow 2.Mucosal ischemia 3.Reduced mucus production 4.Reduced mucosal Prostaglandin production 5.increased gastrin production 6.acid-base abnormalities 7.reflux of bile.
TYPES OF ULCERS
Curling’s ulcers associated with extensive burns
Cushing’s ulcers associated with intracranial pathology and gastric acid hypersecretion
STRESS ULCER MANAGEMENT
- Optimal oxygen delivery to gastric mucosa, avoid hypotension
- Enteral feeding
- Proton pump inhibitors, H2 antagonists: by increasing the pH of gastric contents, there is an increased risk of bacterial colonisation and subsequent nosocomial pneumonia.
- Sucralfate: Aluminium salt of sulphated sucrose and is given in a dose of 1g by NG tube, 6-hourly. It forms a paste at low pH, which preferentially binds to areas of peptic ulceration, thereby providing a physical barrier to the effects of acid. Some investigators have demonstrated a reduction in the rate of nosocomial pneumonias in patients treated with sulcralfate.
MANAGEMENT OF GI BLEED
ECG, NIBP and SpO2 monitoring
Get two wide bore IV access and start RL or NS upto 2 Ls
Support airway: evaluate for the need for airway protection; high volume effective suction must, reduce induction agents dose in hypovolemia
Invasive arterial and central venous pressure monitoring may be necessary in massive bleeds, intubated patients and those with co-morbidities
Insert a Foley for measurement of I/O.
Look for evidence of shock due to severe bleeding: tachycardia,hypotension, ongoing bleed, no response to 2L of crystalloids, HR>90, SBP<100
Rule out hemoptysis and epistaxis
H/O Peptic ulcer disease, NSAID use, alcholism, cirrhosis, coagulation disorders
Order CBC, PT, PTT, BUN, Creatinine, Glucose, Na,K, LFT Cross match 4–6 units of blood as needed
Upright lateral CXR or lateral decubitus abdominal X-rays
Blood should be given promptly if there is persistent haemodynamic instability despite
2 L of crystalloid or colloid, if the initial haemoglobin level is <7 mg/dL, if there is a significant risk of re-bleeding and in those patients with co-morbidities making them unable to tolerate periods of anaemia
Correct coagulopathy
Arrange for endoscopy: both diagnostic / therapeutic
Consider use of vasopressin or octreotide IV, i.v. PPIs.
NSAID INDUCED GASTRIC ULCERS
NSAIDs reduce circulating prostaglandins that are essential in maintaining gastric mucosal integrity. The more an NSAID blocks COX 1, the greater is its tendency to cause peptic ulceration and promote bleeding. Selective COX 2 inhibitors cause less bleeding and fewer ulcers than other NSAIDs
NSAID INDUCED RENAL FAILURE
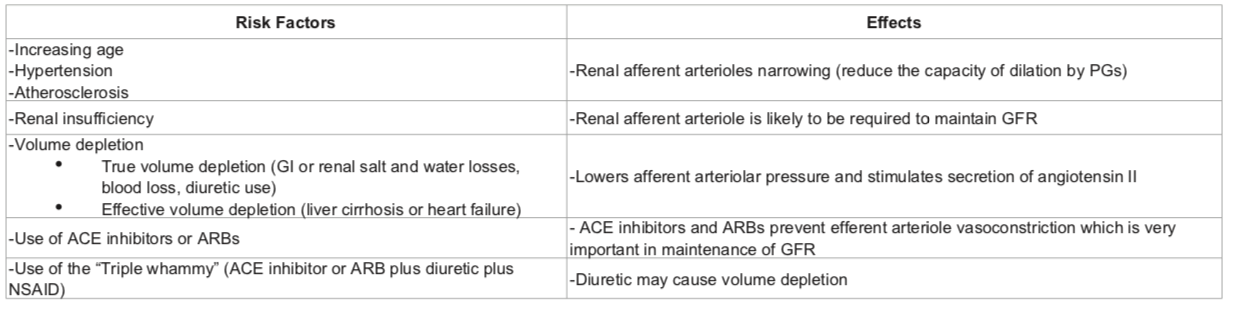
NSAIDs reduce afferent arteriolar blood flow by antagonizing vasodilatory prostaglandins in patients with in patients with risk factors. In this situation, glomerular filtration rate (GFR) drops leading to AKI.
RISK FACTORS: pre-existing renal dysfunction, diabetics, elderly patients, dehydration, decompensated cirrhosis, CHF and patients on ARBs/ACE inhibitors. The mechanism involves reduced levels of prostacyclin, which are required to maintain renal perfusion.
NSAIDs can result in Acute kidney injury (AKI) resulting in the abrupt loss of kidney function, leading to the retention of waste products, electrolyte disturbances, and volume status changes
NSAIDs can also result in Acute interstitial nephritis (AIN) is a renal lesion characterized by a rapid deterioration in kidney function with inflammation and edema of the renal interstitium
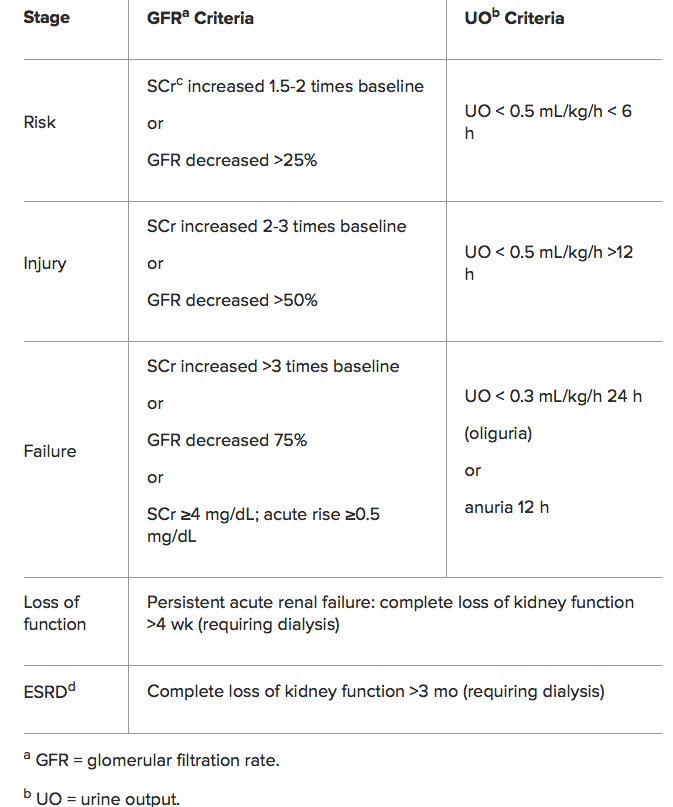
RIFLE Classification for AKI
THIS PATIENT REQUIRES BLOOD TRANSFUSION: ABO INCOMPATIBILITY; WHAT’S IT?
Blood groups result from the different antigens expressed on RBCs. ABO and Rh systems are the most important out of the 29 groups. Early in life persons develop antibodies in plasma against non-self antigens.
ANTIGEN IN RBC | ANTIBODY IN PLASMA |
Group A has A antigen | Group A has antibody to group B |
Group B has B antigen | Group B has antibodies to group A |
Group O has no antigen | Group O has antibody to group A and group B |
Group AB has A and B antigens | Group AB individuals do not have antibody to group A or B. |
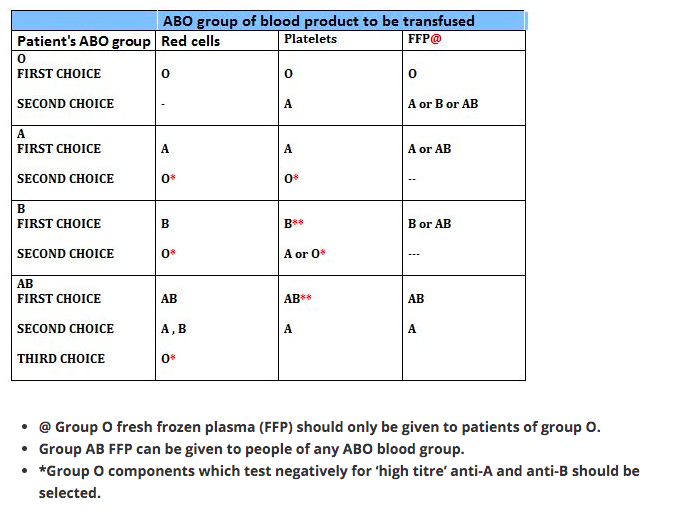
So the blood group AB are ideal recipients, as their plasma doesn’t contain any antibody that can agglutinate the donor blood. In the same way blood group O are ideal donors, as once the antibody containing plasma is removed, it contains no antigens to agglutinate with the antibodies in donor’s blood.
RBC
1 Group O individuals can receive blood from group O donors only ( as the antibodies against A or B in their plasma will react with any A or B antigens which enter the circulation)
2 Group A individuals can receive blood from group A and O donors
3 Group B individuals can receive blood from group B and O donors
4 Group AB individuals can receive blood from AB donors, and also from group A, B and O donors ( as their plasma don’t have any antibodies against any antigens)
PLASMA
In plasma transfusion, group AB plasma can be given to a patient of any ABO group because it contains neither anti-A nor anti-B antibody. Group A plasma (with anti-B) can be given to group O and A patients. Group B plasma to group O and B patients only. Group O plasma (anti-A + anti-B) can be given to group O patients only.
PLATELETS
The Platelet Concentrates transfused must be ABO-identical, or at least ABO-compatible, in order to give a good yield (In an emergency, ABO non-identical units can be used, although the improvement seen in platelet count post-transfusion may be less.) Group O PC can be used for patients with blood groups A, B, and AB ONLY IF, they are resuspended in additive/preservative solutions, or if negative for high titre anti-A/A,B. Rh-negative patients, in particular women of childbearing age, should receive, if possible, RhD-negative PC
Rh BLOOD GROUP
Is the second most important group system. Out of the existing C,D and E antigens, D is the most antigenic one. Anti D antibodies are not normally found in the blood of Rh negative individuals; instead they develop it only when itcomes into contact with Rh positive blood during child birth or inappropriate transfusion. In case of subsequent transfusins or pregnancies with Rh positive blood- this can cause rapid destruction of RhD positive red cells (Hemolytic disease of the newborn in subsequent pregnancies; to prevent this sensitization we should give Rhesus imunoglobulin= Anti-D prophylaxis- to the Rh negative mother who gave birth to an Rh positive baby). FFP does not need to be Rh-compatible. Anti-D prophylaxis is not necessary in Rh D-negative recipients of Rh D-positive FFP.