Tag Archives: edaic
PHENTOLAMINE
- Competitive alpha blocker
- Used in hypertensive emergencies like in pheochromocytoma; also in CRPS
- MOA: 3 times more specific for alpha 1 receptors than alpha 2. Doesn’t bind covalently to receptors; hence reversible. Also has beta agonistic and anti-serotoninergic activity
- Comes as pale yellow solution 10 mg/mL; dose is 1–5 mg titrated to effect
- CVS: Vasodilation and reduce BP, improve coronary artery perfusion, reduce pulmonary artery pressures. Alpha 2 blockade enhances noradrenaline release causing increase in HR and CO
- RESPIRATORY SYSTEM: Increase FEV1, increase secretions, prevents bronchospasm caused by histamine release
- Other Side Effects: Nasal congestion, Hypoglycemia (Causes insulin secretion)
PHENOXYBENZAMINE
- Phenoxybenzamine is a haloalkylamine. Used as a vasodilator in hypertensive emergencies like in pheochromacytoma; also used in the management of CRPS. Used in the management of intraarterial injection of thiopentone too.
- It is a non-selective alpha blocker. It binds covalently to the receptors. So it behaves like a competitive irreversible antagonist.New receptors must be synthesised to overcome drug effect. Blockade of alpha 2 receptors increases the amount of noradrenaline released: produces tachycardia. Partial agonist at 5-HT2 receptors
- Available as capsules of 10 mg and solution: 50 mg/mL for iv use. Oral dose is 10–60 mg per day in divided doses and IV: 10–40 mg over 1 hour. Effects may lasts for 3–4 days
- CVS: Vasodilation and fall in BP, but tachycardia increase CO
- CNS: Sedation. Rapid infusion can cause seizures
Describing a drug in exams
- Introduction, Group
- Uses
- Chemical characteristics, Presentation
- Mechanism of action
- Routes of administration and dosing
- Pharmacokinetics: Absorption, Distribution, Metabolism, Excretion
- Pharmacodynamics: CNS/CVS/GIT/RENAL/METABOLIC/MUSCULOSKELETAL etc
- Side effects
- Special points
VIVA SCENE: THROMBOELASTOGRAPHY (TEG) AND OTHER QUESTIONS
TEG is a relatively new modality for monitoring coagulation which is very useful during management of trauma and also in the perioperative scenario..
BASIS:
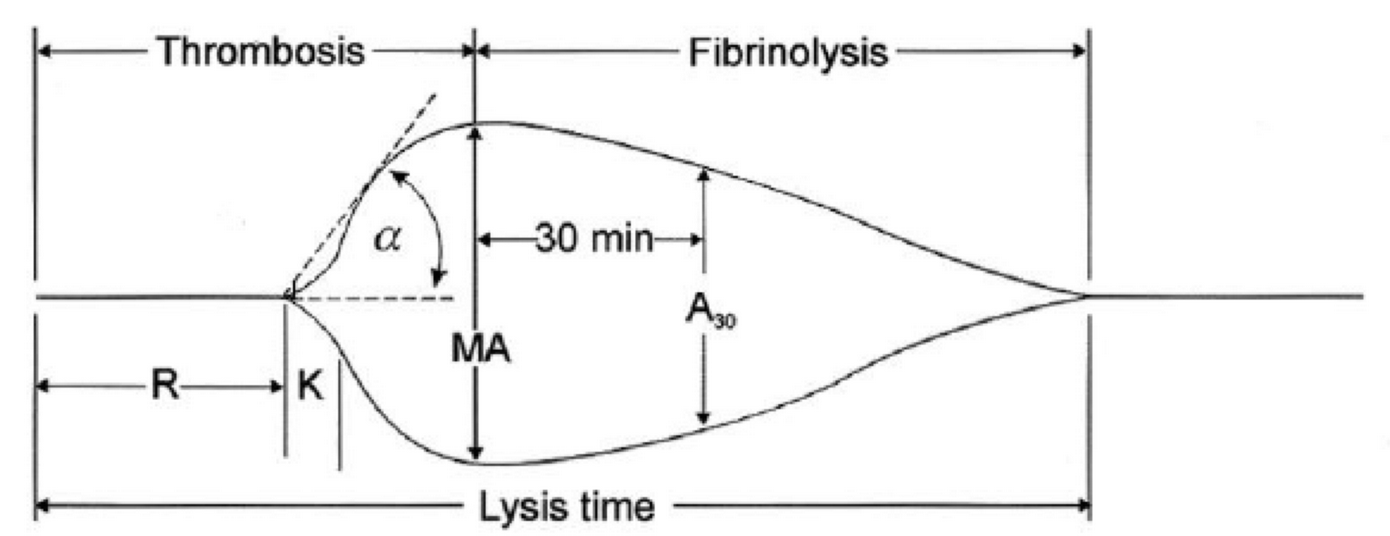
- The 2 main components of the TEG machine are a cup and a pin. Whole blood is mixed with the activating agent kaolin as well as calcium. The cup then oscillates around the pin slowly, at a rate of 6 times per minute, to mimic natural blood flow in vivo and activate the clotting cascade. As the clot forms, the torque between the cup and pin is transduced and measured, creating a curve. As the clot breaks down and torque decreases, the tracing converges to represent this.
- The different parameters of the curve are then measured to assess current coagulation status.
- Of the 4 types of TEG assays available, the most common is the rapid TEG. The use of an activator in rapid TEG standardizes the TEG test and speeds up the rate at which clotting takes place, thus making results available more quickly.
INTERPRETATION
-
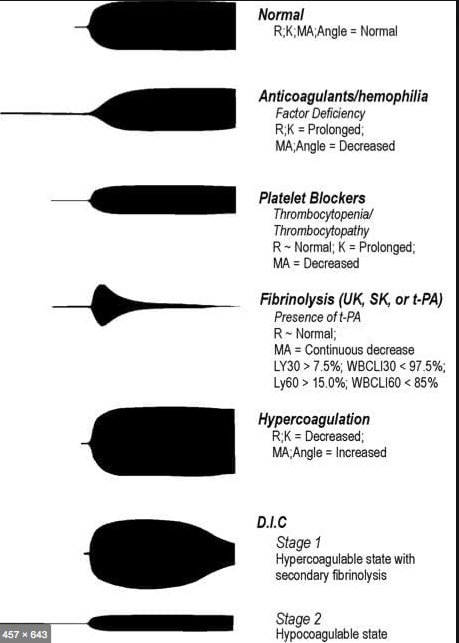
R(sec): The first measurement of note is the reaction time (R time). This is the time interval from the start of the test to the initial detection of the clot. Normal R values range between 7.5 and 15 minutes. A prolonged R time may indicate hemodilution or clotting factor deficiencies. The treatment for prolonged R time is to administer FFP as it contains all factors of the coagulation cascade, without further coagulant hemodilution. A shortening of R time (< 3 minutes) occurs in hypercoagulable states. Examples would be patients with early disseminated intravascular coagulation (DIC) or septicemia. In these situations, free thrombin is released into the circulating blood, triggering the clotting mechanisms but the patient later begins to bleed because of exhaustion of clotting factors.
-
K (sec) and Angle α (°): The clot strength is measured by these 2 variables in TEG. The K value measures the interval between the R time and the time when the clot reaches 20 mm. Normal K values range between 3 and 6 minutes. Prolongation of the K value with normal platelet count indicates inadequate amounts of fibrinogen to form fibrin. The treatment for prolonged K value is therefore to administer fibrinogen/cryoprecipitate. The α angle measures a line tangent to the slope of the curve during clot formation.The alpha angle represents the thrombin burst and conversion of fibrinogen to fibrin. Normal α value is between 45° and 55°. A longer K value causes a shallow or more acute angle (<45°), while a shorter K value causes a steeper α angle (>45 °). An angle α <45° suggests a less vigorous association of fibrin with platelets. In this case, treatment begins much higher on the coagulation cascade, with the replacement of both fibrinogen and factor VIII. Thus, these patients can be treated with the administration of cryoprecipitate. Shortening of the K-value indicates a very quick formation of clot, potentially due to hypercoagulability or inappropriate consumption of coagulation factors. A shortened K value also corresponds to a steeper α (>45°). The treatment for shortened K and steeper α is anticoagulation therapy
- MA (mm): Maximum amplitude is a measurement of maximum clot strength and provides information on both fibrinogen and platelet function. As the clot develops and increases in tensile strength due to platelet activation and binding to fibrin, the tracing increases it’s MA or appears to widen. Normal values are between 50–60 mm. 80% of the MA is derived from platelet function whereas the remaining 20% is derived from fibrin. A low MA value is indicative of low clot strength, which can be caused by decreased fibrinogen levels, low platelet counts, or decreased platelet function. (i) Paired with a prolongation of K value, this could be a sign of the need for cryoprecipitate. (ii) Administration of platelets may be avoided when a low platelet count is combined with a normal MA value (=platelet function is normal) (iii) Treatment with platelets may be indicated for patients with a low MA value (=low platelet function) and normal platelet count. (iv) High MA will occur in the setting of hyperactivity of platelets, and MA above 75 mm indicates a prothrombotic state. In this case, treating with an anticoagulant would be helpful
- Shear Elastic Modulus Strength, G value or G: is a measure of clot strength or clot firmness, and is calculated based on the amplitude value (A) until the maximum amplitude (MA) is reached. It is the single most important value of the entire assay because it represents the overall function or effectiveness of the clot. Normal G values are between 5.3 and 12.4 dynes/cm2. A G value >10 dynes/cm2 indicates increased risk of thrombosis. Treatment for high G is accomplished by the use of platelet inhibitors such as Clopidogrel or Aspirin. Aspirin is usually not preferred because it inhibits platelet adherence rather than platelet aggregation. A G <5 dynes/cm2 places a patient at increased risk of hemorrhage
- As time progresses during the TEG assay, the tracing will remain at maximal amplitude for a period of time, after which clot lysis begins. Normally, lysis continues for a period of up to 15 minutes. A computerized algorithm automatically estimates the percentage of lysis occurring over time. This is called the Estimated Percentage of Lysis or EPL. After 30 minutes, EPL becomes EPL30 or succinctly LY30 (i.e. percentage of lysis at 30 minutes). Both the EPL and LY30 are measurements of excessive fibrinolysis since they measure the percentage decrease in amplitude after MA. An EPL between 7.5 and 15%, when accompanied by a very high G, reflects a hyperfibrinolytic and hypercoagulable state typical of patients with early DIC. A very high EPL or LY30 (>20%) may indicate the need for antifibrinolytic therapy, such as the use of transexamic acid or aminocaproic acid. LY30 is also useful for patients undergoing thrombolytic drug therapy. This can be observed by rapid curve convergence.
Ref: Thromboelastography: Clinical Application, Interpretation, and Transfusion Management, Shawn Collins et al AANA Journal Course, 2016
HOW DO WE TEST CLOTTING?
- By doing tests like aPTT, PT & INR, Platelet Count, ACT, Bleeding Time, fibrinogen and factor levels, TEG etc
- The aPTT and INR use different reagents to measure the time to form a clot in vitro after platelet-poor plasma from blood collected in a calcium chelating tube, is recalcified
- The aPTT is prolonged with the deficiency of factors of the intrinsic pathway: Fs 8,9,11,12. Also the factors involved in the common pathway (Fs 1,2,10)e.g. Heparin therapy, DIC, liver disease
- The INR is prolonged especially with deficiency of F 7; but also with deficiency of Fs 1,2,5,10 e.g. warfarin therapy, vitamin K deficiency, DIC, liver disease
- N.B Warfarin inhibits the gamma carboxylation of vitamin K dependent factors 2,7,9,10
- BLEEDING TIME :Duke’s method: Sterilize the finger tip using rectified spirit and allow to dry. Make a sufficiently deep prick using a sterile lancet, so that blood comes out freely without squeezing. Note the time (start the stop-watch) when bleeding starts. Mop the blood by touching the finger tip with a filter paper. This is repeated every 15 seconds, each time using a fresh portion of the filter paper, till bleeding stops. Note the time (stop the stop-watch). Normal value is upto 4 minutes.
- CLOTTING TIME: Capillary tube method: (Wright’s method). Under sterile precautions make a sufficiently deep prick in the finger tip. Note the time when bleeding starts (start the stop watch). Touch the blood drop at the finger tip using one end of the capillary tube kept tilted downwards. The tube gets easily filled by capillary action. After about two minutes start snapping off small lengths of the tube, at intervals of 15 seconds, each time noting whether the fibrin thread is formed between the snapped ends. Note the time (stop the stop watch) when the fibrin thread is first seen. Clotting time is the interval between the moment when bleeding starts and the moment when the fibrin thread is first seen.
Normal value is 3 to 10 minutes.
- Bleeding time depends on the integrity of platelets and vessel walls, whereas clotting time depends on the availability of coagulation factors
VIVA SCENE: CARBON MONOXIDE (CO) POISONING
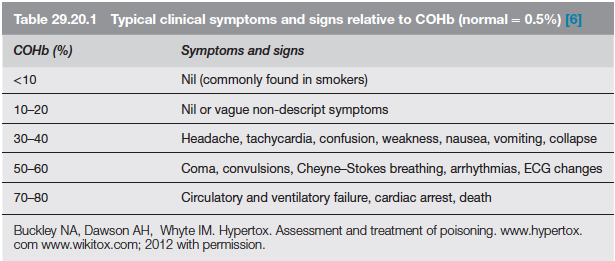
AETIOLOGY: Carbon monoxide is produced by incomplete combustion and is found in car exhaust, faulty heaters, fires and in industrial settings. Carboxyhaemoglobin (COHb) concentrations in cigarette smokers range as high as 10%.
MECHANISM: Binds to Hb with 210 times affinity than O2: so reduce the O2 carrying capacity of blood. Also disrupts oxidative metabolism, binds to myoglobin and cytochrome oxidases, causes lipid peroxidation. Final result is tissue hypoxia. Severity depends on the duration of exposure, CO levels and patients pre-event health status: pre-existing cerebral disease, cardiac failure, hypovolemia and anemia increase toxicity
DIFFERENTIAL DIAGNOSIS: Cyanide poisoning ( suspected when CNS effects are out of proportion with COHb concentrations and if there is a marked lactic acidosis)
EVALUATION & MANAGEMENT:
- ABC approach
- Secure the airway; if GCS<8, consider intubation
- Stabilize respiration: consider mechanical ventilation or CPAP
- Get intravenous access
- Send samples for estimation of Hb(?anemia), electrolytes (dyselectrolytemias worsen the cardiac toxicity), COHb levels (to confirm diagnosis; useless in prognosis), blood sugar, ABG and cardiac enzymes. Take an ECG.
- Metabolic acidosis due to lactate give a clue to the extend of ischemia. Net effect of metabolic acidosis may be beneficial on O2 delivery; but treated if pH<7
- Patient discouraged from activity
- 100% O2 reduces the half life of COHb from 4 hours (in ambient air) to 40 minutes. 4 to 6 h of 100% normobaric oxygen will remove over 90% of the carbon monoxide. Oxygen toxicity is unlikely with less than 24 h treatment
- When immediately available, hyperbaric oxygen (HBO) should be considered with serious CO poisoning. Oxygen at 2–3 atmospheres will further reduce the half-life of COHb to about 20 min but, more importantly, it causes very rapid reversal of tissue hypoxia due to oxygenation of tissue from oxygen dissolved in the plasma. Some clinicians implement it based on the presence of any of the following: history of loss of consciousness, abnormal neuropsychiatric testing or neurological signs, pregnancy. COMPLICATIONS OF HBO: decompression sickness, rupture of tympanic membranes, damaged sinuses, oxygen toxicity
- CO PRODUCTION WITH SODALIME USE: Occurs when inhalational agents with CHF2 moiety such as desflurane, enflurane, and isoflurane are used with desiccated soda lime granules that was left unused for a long time. Can be significant in smokers especially when very low flows are used. Factors increasing the production of CO include° Type of inhaled anaesthetic agent (magnitude of CO production from greatest to least is desflurane > enflurane > isoflurane > sevoflurane)° High absorbent dryness ° Type of absorbent (at a given water content, baralyme produces more CO than soda lime)° Increased temperature° Higher anaesthetic concentration
VIVA SCENE: GASTRIC ULCER BLEED AND OTHER QUESTIONS
STRESS ULCER RISK FACTORS
Stress ulceration in intensive care patients is relatively common (approaching 90% by day 3 with no prophylaxis), although the incidence of clinically important GI bleeding is less than 2%. There are six major risk factors: 1.Respiratory failure requiring ventilation for >48 hours 2.Coagulopathy 3.Sepsis 4.Hypotension 5.Hepatic failure 6.Renal failure
STRESS ULCER PATHOPHYSIOLOGY
- Impaired mucosal blood flow 2.Mucosal ischemia 3.Reduced mucus production 4.Reduced mucosal Prostaglandin production 5.increased gastrin production 6.acid-base abnormalities 7.reflux of bile.
TYPES OF ULCERS
Curling’s ulcers associated with extensive burns
Cushing’s ulcers associated with intracranial pathology and gastric acid hypersecretion
STRESS ULCER MANAGEMENT
- Optimal oxygen delivery to gastric mucosa, avoid hypotension
- Enteral feeding
- Proton pump inhibitors, H2 antagonists: by increasing the pH of gastric contents, there is an increased risk of bacterial colonisation and subsequent nosocomial pneumonia.
- Sucralfate: Aluminium salt of sulphated sucrose and is given in a dose of 1g by NG tube, 6-hourly. It forms a paste at low pH, which preferentially binds to areas of peptic ulceration, thereby providing a physical barrier to the effects of acid. Some investigators have demonstrated a reduction in the rate of nosocomial pneumonias in patients treated with sulcralfate.
MANAGEMENT OF GI BLEED
ECG, NIBP and SpO2 monitoring
Get two wide bore IV access and start RL or NS upto 2 Ls
Support airway: evaluate for the need for airway protection; high volume effective suction must, reduce induction agents dose in hypovolemia
Invasive arterial and central venous pressure monitoring may be necessary in massive bleeds, intubated patients and those with co-morbidities
Insert a Foley for measurement of I/O.
Look for evidence of shock due to severe bleeding: tachycardia,hypotension, ongoing bleed, no response to 2L of crystalloids, HR>90, SBP<100
Rule out hemoptysis and epistaxis
H/O Peptic ulcer disease, NSAID use, alcholism, cirrhosis, coagulation disorders
Order CBC, PT, PTT, BUN, Creatinine, Glucose, Na,K, LFT Cross match 4–6 units of blood as needed
Upright lateral CXR or lateral decubitus abdominal X-rays
Blood should be given promptly if there is persistent haemodynamic instability despite
2 L of crystalloid or colloid, if the initial haemoglobin level is <7 mg/dL, if there is a significant risk of re-bleeding and in those patients with co-morbidities making them unable to tolerate periods of anaemia
Correct coagulopathy
Arrange for endoscopy: both diagnostic / therapeutic
Consider use of vasopressin or octreotide IV, i.v. PPIs.
NSAID INDUCED GASTRIC ULCERS
NSAIDs reduce circulating prostaglandins that are essential in maintaining gastric mucosal integrity. The more an NSAID blocks COX 1, the greater is its tendency to cause peptic ulceration and promote bleeding. Selective COX 2 inhibitors cause less bleeding and fewer ulcers than other NSAIDs
NSAID INDUCED RENAL FAILURE
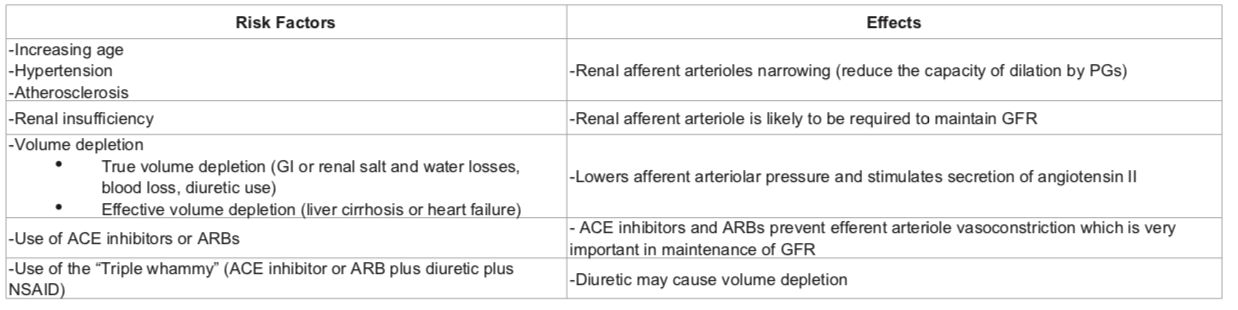
NSAIDs reduce afferent arteriolar blood flow by antagonizing vasodilatory prostaglandins in patients with in patients with risk factors. In this situation, glomerular filtration rate (GFR) drops leading to AKI.
RISK FACTORS: pre-existing renal dysfunction, diabetics, elderly patients, dehydration, decompensated cirrhosis, CHF and patients on ARBs/ACE inhibitors. The mechanism involves reduced levels of prostacyclin, which are required to maintain renal perfusion.
NSAIDs can result in Acute kidney injury (AKI) resulting in the abrupt loss of kidney function, leading to the retention of waste products, electrolyte disturbances, and volume status changes
NSAIDs can also result in Acute interstitial nephritis (AIN) is a renal lesion characterized by a rapid deterioration in kidney function with inflammation and edema of the renal interstitium
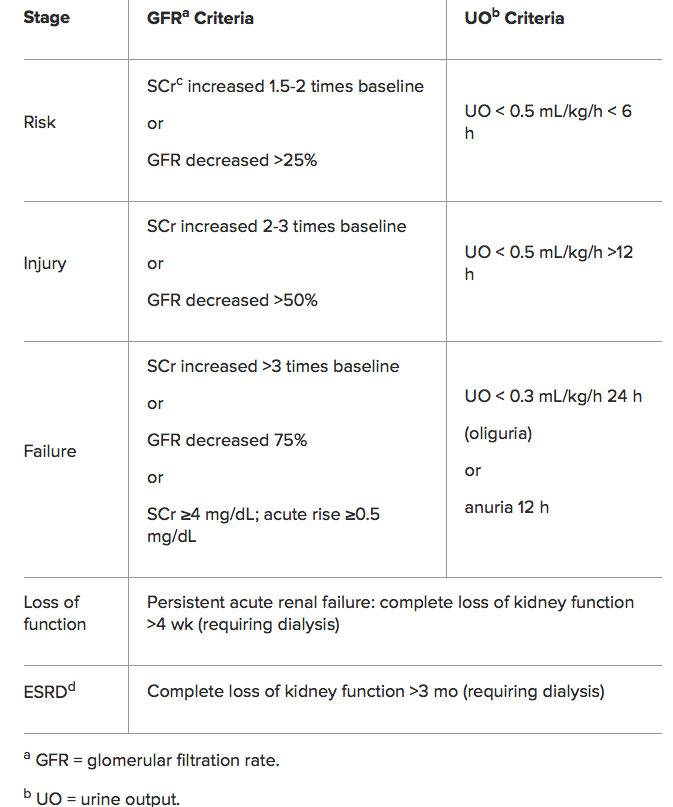
RIFLE Classification for AKI
THIS PATIENT REQUIRES BLOOD TRANSFUSION: ABO INCOMPATIBILITY; WHAT’S IT?
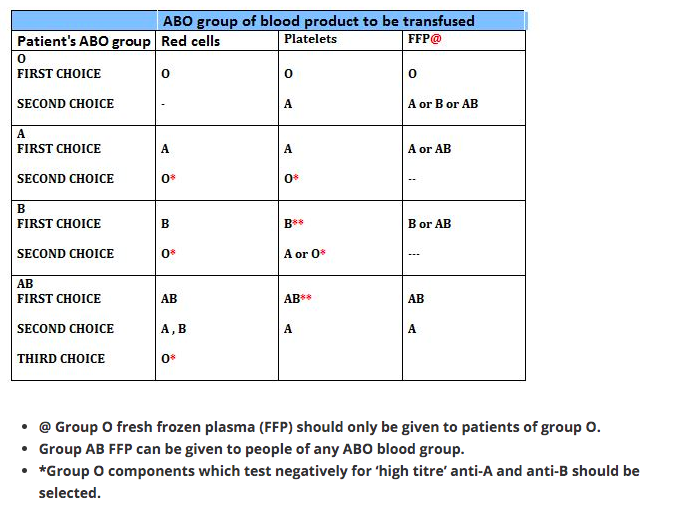
Blood groups result from the different antigens expressed on RBCs. ABO and Rh systems are the most important out of the 29 groups. Early in life persons develop antibodies in plasma against non-self antigens.
ANTIGEN IN RBC | ANTIBODY IN PLASMA |
Group A has A antigen | Group A has antibody to group B |
Group B has B antigen | Group B has antibodies to group A |
Group O has no antigen | Group O has antibody to group A and group B |
Group AB has A and B antigens | Group AB individuals do not have antibody to group A or B. |
So the blood group AB are ideal recipients, as their plasma doesn’t contain any antibody that can agglutinate the donor blood. In the same way blood group O are ideal donors, as once the antibody containing plasma is removed, it contains no antigens to agglutinate with the antibodies in donor’s blood.
RBC
1 Group O individuals can receive blood from group O donors only ( as the antibodies against A or B in their plasma will react with any A or B antigens which enter the circulation)
2 Group A individuals can receive blood from group A and O donors
3 Group B individuals can receive blood from group B and O donors
4 Group AB individuals can receive blood from AB donors, and also from group A, B and O donors ( as their plasma don’t have any antibodies against any antigens)
PLASMA
In plasma transfusion, group AB plasma can be given to a patient of any ABO group because it contains neither anti-A nor anti-B antibody. Group A plasma (with anti-B) can be given to group O and A patients. Group B plasma to group O and B patients only. Group O plasma (anti-A + anti-B) can be given to group O patients only.
PLATELETS
The Platelet Concentrates transfused must be ABO-identical, or at least ABO-compatible, in order to give a good yield (In an emergency, ABO non-identical units can be used, although the improvement seen in platelet count post-transfusion may be less.) Group O PC can be used for patients with blood groups A, B, and AB ONLY IF, they are resuspended in additive/preservative solutions, or if negative for high titre anti-A/A,B. Rh-negative patients, in particular women of childbearing age, should receive, if possible, RhD-negative PC
Rh BLOOD GROUP
Is the second most important group system. Out of the existing C,D and E antigens, D is the most antigenic one. Anti D antibodies are not normally found in the blood of Rh negative individuals; instead they develop it only when itcomes into contact with Rh positive blood during child birth or inappropriate transfusion. In case of subsequent transfusins or pregnancies with Rh positive blood- this can cause rapid destruction of RhD positive red cells (Hemolytic disease of the newborn in subsequent pregnancies; to prevent this sensitization we should give Rhesus imunoglobulin= Anti-D prophylaxis- to the Rh negative mother who gave birth to an Rh positive baby). FFP does not need to be Rh-compatible. Anti-D prophylaxis is not necessary in Rh D-negative recipients of Rh D-positive FFP.
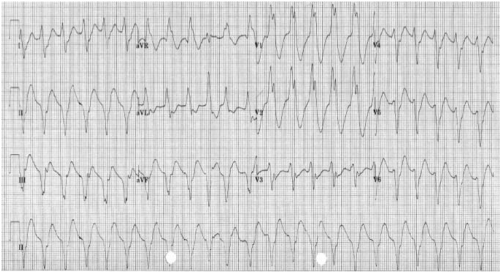
Broad Complex Tachycardias
A broad complex tachycardia has a QRS complex greater than 0.12 seconds. They are usually ventricular in origin, but can also be supraventricular with aberrant conduction. Other possible causes for broad complex tachycardias include atrial fibrillation with ventricular pre-excitation, i.e. patients with Wolff–Parkinson–White (WPW) syndrome, or torsades de pointes (polymorphic VT).
Broad complex tachycardia is therefore due to SVT with aberrancy or Ventricular Tachycardia (VT), and differentiating between the two can be challenging. However, there are a few pathognomonic ECG features that diagnose VT
1.Atrio-ventricular (AV) dissociation. There is a higher ventricular rate than atrial rate (more QRS complexes than P-waves). This can only occur if the ventricular rate is autonomous and no longer under control of the SA node.
2.Capture beats: There is an isolated narrow complex amongst a train of broad complexes. This represents a normally conducted P-wave via the AV node and an intact His-Purkinje system indicating there is no underlying bundle branch block. Therefore, the train of broad complexes are ventricular in origin (i.e. VT).
3.Fusion beats: A normally conducted P-wave may fuse with a simultaneous ventricular beat causing a complex halfway between the appearance of a normal QRS and a broad complex.
4.VT is more likely in patients with a prior history of MI.
5.VT complexes are usually very broad (> 160 ms) due to a very abnormal path taken by the depolarisation wave from the VT focus.
6.The time from R-wave onset to the nadir of the S-wave is prolonged (> 100 ms) in VT, again representing an abnormal activation path through the ventricle.
7.Extreme left axis deviation and positive aVR are more common in VT, as the ventricles are depolarised in the opposite direction to normal conduction.
8.Failure to respond to iv adenosine
9.The absence of typical RBBB or LBBB patterns suggests VT. For example, an RSR pattern in V1 with a taller first R-wave suggests VT (in RBBB the first R-wave is caused by septal depolarisation and is therefore smaller than the second R-wave, which is caused by depolarisation of the RV).
SVT with aberrancy is more likely if previous ECGs demonstrate an accessory pathway or a bundle branch block with identical morphology to the broad complex tachycardia. When in doubt, treat as VT
TORSADES DE POINTES
Is a specific variant of ventricular tachycardia (VT). It has a classic undulating pattern with variation in the size of QRS complex. It is caused by a prolonged QT interval and can precipitate VF and sudden death
QT PROLONGATION: CAUSES
Tricyclic antidepressants, flecainide and quinidine; Hypocalcemia; Acute myocarditis
VENTRICULAR TACHYCARDIA(VT) AND VENTRICULAR FIBRILLATION (VF)
VT is a broad complex tachycardia, defined as a run of at least three consecutive ventricular ectopic beats, at a rate of >120 bpm. Can arise from a single or multiple foci or from a reentry circuit. There may be capture or fusion beats, where a normally conducted beat will join an ectopic beat travelling in the opposite direction
CAUSES: Acute MI, degeneration of other arrhythmias, electrolyte abnormalities etc
VF describes an ECG which is random and chaotic with no identifiable QRS complexes that is incompatible with life and need immediate provision of ACLS with prompt delivery of DC shock. Others: Amiodarone, Lidocaine, beta blockers, Implantable cardioverter defibrillators
MANAGEMENT
For VT treat with amiodarone 300 mg IV followed by 900 mg over 24 hours. If the arrhythmia is known to be supraventricular, treat as a narrow complex tachycardia.
An irregular broad complex tachycardia is most likely to be atrial fibrillation with bundle branch block, and should be treated as narrow complex atrial fibrillation
In a stable patient who is known to have WPW, the use of amiodarone is probably safe. Adenosine, digoxin, verapamil and diltiazem must be avoided, as these drugs block the AV node and will cause a relative increase in pre-excitation
Torsades de pointes is treated by stopping all drugs known to prolong the QT interval and correcting electrolyte abnormalities. Magnesium sulphate (2 g IV over 10 minutes) should also be given. Such patients may require ventricular pacing. If the patient’s condition deteriorates proceed to synchronised electrical cardioversion or, if the patient is pulseless, commence the ALS algorithm
Ventricular bigeminy
Ventricular bigeminy is associated with endotracheal intubation (a sympathoadrenal response). Given time the bigeminy will disappear, but if it does not intravenous
lidocaine (50–100 mg) may be helpful
Narrow Complex Tachycardias
Narrow complex tachyarrhythmias have a QRS duration <0.12 seconds. They arise above the bundle of His.
NARROW COMPLEX TACHYCARDIA
As narrow complex tachycardias involve ventricular activation through the normal His-Purkinje system, they must originate within the atria and are therefore often referred to as supraventricular tachycardia (SVT). There are five common types of SVT. They are: Atrial tachycardia, Atrial fibrillation, Atrial flutter, Atrioventricular nodal
re-entry tachycardia, Atrioventricular re-entry tachycardia. When faced with an ECG of narrow complex tachycardia, (i) we should examine the P-wave and (ii) check the QRS regularity
SINUS ARRHYTHMIA/TACHYCARDIA/BRADYCARDIA (from SA Node)
ATRIAL FIBRILLATION
There is completely disorganised atrial activity, with P-waves replaced by an irregular baseline due to fibrillation waves, and QRS complexes occur in an irregularly irregular fashion (Please the post on AF)
ATRIAL FLUTTER
There is a self-perpetuating wave of atrial depolarisation usually circulating within the right atrium, causing regular, saw-toothed flutter waves at 300 bpm and QRS complexes every second, third, or fourth flutter wave. We can see classical sawtooth flutter waves.Drug control of the ventricular rate is not often successful.
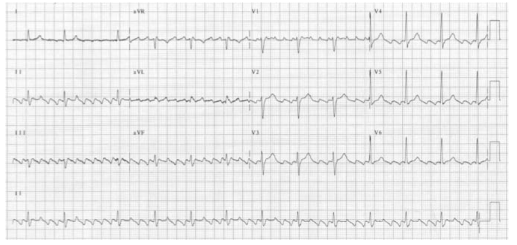
ATRIAL TACHYCARDIA
There is an abnormal atrial focus driving the ventricular rate. This rhythm can be difficult to distinguish from sinus tachycardia, but P-wave morphology and axis is usually abnormal. If the atrial focus is close to the AV node, a junctional tachycardia may occur and P-waves may be absent.
In case of Atrial tachycardia with AV block after halting glycoside therapy (and ensuring normokalaemia), lidocaine 1 mg kg−1 IV is the drug
of choice. Alternatively DC cardioversion or atrial
pacing may be effective.
ATRIO VENTRICULAR NODAL REENTRY TACHYCARDIA (AVNRT)
This is the commonest type of paroxysmal supraventricular tachycardia (PSVT). It is often seen in people without any heart disease, and is usually benign. There is a rapid reentry circuit within the AV node resulting in simultaneous atrial and ventricular depolarisation. The P-wave is usually buried within the QRS or ST-segment. There will be fast regular narrow complex tachycardia, and P-waves can be seen buried in the terminal portion of the QRS complex which may easily be mistaken for a second, small R-wave. The very close proximity of the QRS and P-waves implies near simultaneous depolarisation of atria and ventricles.
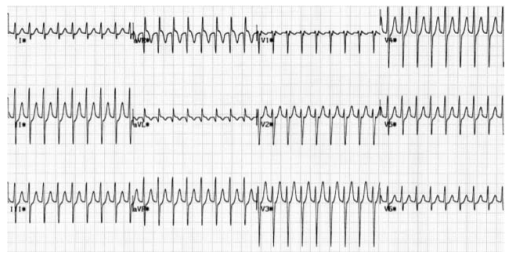
ATRIO VENTRICULAR REENTRY TACHYCARDIA (AVRT)
This occurs in patients with WPW, and is usually benign unless there is coexisting structural heart disease. There is an accessory pathway bridging the atria and ventricles allowing antegrade conduction down the AV node (causing a narrow QRS) and retrograde conduction back to the atria via the accessory pathway. Since the depolarisation wave takes time to complete this circuit, the P-wave occurs after the QRS complex and is often buried within the T-wave. AVRT can occur with antegrade conduction to the ventricles via the accessory pathway, but this will result in ventricular depolarisation via an abnormal route and consequently a broad QRS. In sinus rhythm, antegrade conduction via the accessory pathway produces a short PR interval (as the normal delay in the AV node is avoided) and the abnormal activation of the ventricles produces a slurred upstroke
in the QRS called a delta wave. The QRS complex is said to be pre-excited and can be associated with repolarisation abnormalities. There are seven sinus beats followed by a ventricular ectopic beat that conducts to the atria retrogradely through the atrioventricular node and then returns to the ventricles via the accessory pathway. This cycle repeats and triggers a broad complex tachycardia. (Please see post on ‘WPW Syndrome’ also).
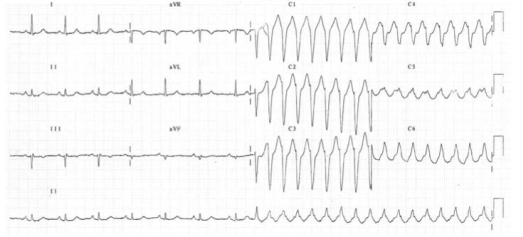
An unstable patient presenting with a regular narrow complex tachycardia should be treated with electrical cardioversion. If this is not immediately available, adenosine should be given as a first-line treatment. A stable patient presenting with a regular narrow complex tachycardia should initially be treated by vagal
manoeuvres such as carotid sinus massage or the Valsalva manoeuvre, as these will terminate up to a quarter of episodes of PSVT. Carotid sinus massage should be avoided
in the elderly, especially if a carotid bruit is present, as it may dislodge an atheromatous plaque and cause a stroke
Management
A stable patient presenting with a regular narrow complex tachycardia should initially be treated by vagal manoeuvres such as carotid sinus massage or the Valsalva manoeuvre, as these will terminate most episodes of PSVT. Carotid sinus massage should be avoided in the elderly, especially if a carotid bruit is present, as it may dislodge an atheromatous plaque and cause a stroke. If the tachycardia persists and is not atrial flutter, 6 mg of adenosine should be given as an IV bolus, followed by a 12 mg bolus if no response. A further 12 mg bolus of adenosine may be given if the tachycardia persists. Vagal manoeuvres or adenosine will terminate almost all AVNRTs or AVRTs within seconds, and therefore failure to convert suggests an atrial tachycardia such as atrial flutter. If adenosine is contraindicated, or fails to terminate a narrow complex tachycardia, without first demonstrating it as atrial flutter, give a calcium-channel blocker, e.g. verapamil 2.5–5 mg IV over two minutes. Atrial flutter should be treated by rate control with a beta-blocker.
An irregular narrow complex tachycardia is most likely to be atrial fibrillation (AF) with an uncontrolled ventricular response, but may also be atrial flutter with variable block. If the patient is unstable, synchronised electrical cardioversion should be used to treat the arrhythmia
ATRIAL FIBRILLATION (AF) AND THE ANESTHESIOLOGIST
Atrial fibrillation(AF) is a supra-ventricular arrhythmia characterized by the complete absence of co-ordinated atrial contractions. There will not be any discernable p-waves.
The ventricular response rate depends on the conduction of the AV node.
WHAT IS THE DIFFERENCE BETWEEN ATRIAL FIBRILLATION AND ATRIAL FLUTTER
Flutter is a more organised and regular form of atrial activity and classically with an atrial rate of 300 bpm. ‘Saw toothed’ flutter waves are present on the ECG. The ventricular response depends on conduction through the AV node. The classic ECG has 2:1 block, hence a ventricular rate of 150 bpm
CAUSES OF AF IN THE PERIOPERATIVE SETTING
Electrolyte abnormalities especially low potassium or magnesium
Withdrawal of beta blockers
Following cardiac surgery.
ASD or mitral valve disease
Ischaemic heart disease
Thyrotoxicosis
Excess caffeine or alcohol (acute or chronic)
Pulmonary embolism
Pneumonia
Pericarditis
In the context of major vascular surgery, systemic inflammation,hypovolemia and a heightened adrenergic state are likely to play a major role.
WHAT IS LONE AF?
‘Lone AF’ is AF in the absence of any demonstrable medical cause, but this is not usually diagnosed in the peri-operative period. So beta blockers will be efficacious in this setting.
WHAT ARE THE PROBLEMS AF CAN POSE?
Loss of the atrial ‘kick’ as it contracts and empties into the LV can reduce the CO by 10%–20% with a normal ventricle (reduced by 40%–50% in those with a ‘stiff’ ventricle as in
diastolic dysfunction, aortic stenosis etc). The disorganised contractions of the atria cause stasis of blood and the risk of thromboembolism. There is a 3%–7% annual risk of
thromboembolic CVA
AF- EVALUATION
*History *Assessment of volume status and electrolytes *ECG: This will also help to exclude acute ischaemia. *The pulse will be irregularly irregular. *No ‘a wave’ in the jugular venous pulsation as this is caused by sinus atrial contraction. *Chaotic atrial activity can be seen on echocardiography.
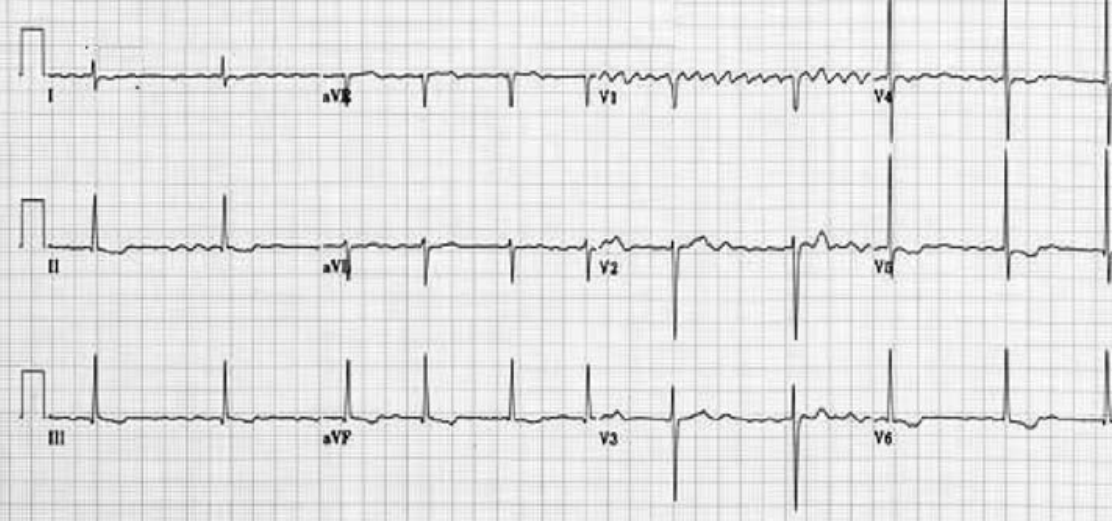
READ THIS ECG

MNEMONIC ‘RIAS QRST’ (Rate & Rhythm, Intervals, Axis, QRS & ST segment T wave)
The rate is 78 bpm; the rhythm is irregularly irregular. There are flutter waves seen in the V1 rhythm strip. The axis is normal (There is borderline LVH by voltage criteria). There are no Q waves and the QRS width is normal. There is evidence of infero-lateral ischaemia shown by the inverted and biphasic T waves in this territory (II, III, aVF and V3−V6).
MANAGEMENT OF AF
Assess for cardiovascular compromise and resuscitate simultaneously if needed. Oxygen should be administered, continuous ECG monitoring instituted and IV access secured.
If the patient is unstable, synchronised electrical cardioversion should be used to treat the arrhythmia.
In a stable patient, treatment options include: Rate control by drug therapy. Drugs used to control the heart rate include beta-blockers, digoxin, magnesium, the non-dihydropyridine calcium channel blockers (verapamil or diltiazem) or a combination of these.
Rhythm control by amiodarone to encourage cardioversion: Amiodarone is given as a 300 mg IV bolus, followed by 900 mg IV over 24 hours.
Rhythm control by electrical cardioversion: This is more likely to restore sinus rhythm than chemical cardioversion.
Treatment to prevent complications. Patients who are in AF are at risk of atrial thrombus formation and should be anticoagulated
Patients who have pre excitation syndromes with an accessory conduction pathway between the atria and ventricles (such as in the Wolff–Parkinson–White syndrome) should not be given AV node blocking drugs if they develop an SVT. This will promote the atrial impulses to travel directly to the ventricle at up to 300 bpm via the accessory pathway. The drugs of choice are amiodarone, flecainide or procainamide.
BEFORE PROCEEDING WITH DC CARDIOVERSION FOR AF, WHAT ALL THINGS SHOULD BE CONSIDERED?
- Cardioversion should only be attempted without anticoagulation if the duration of the AF is less than 48 hours. If the duration is unknown or longer than this, 3–4 weeks of anticoagulation (INR 2–3) is required to reduce the incidence of clot embolisation. If there is a contra-indication to anticoagulation, or if the cardioversion is deemed necessary more urgently, then an echocardiogram is needed to exclude thrombus in the atrium and atrial appendage
- When did the AF start (history of palpitations or recording on monitor): is it acute or chronic?
- What is the likelihood of an atrial thrombus which could be embolised by
cardioversion? - What is the ventricular rate now? – may need pacing after cardioversion if
the rate is below 60 bpm - Has there been an ischaemic episode?
ANESTHESIA FOR CARDIOVERSION
This should be done in a critical care or operating room area with the usual preparation, equipment and assistance needed for any routine anaesthetic. Someone independent should be present to perform the defibrillation, preferably with a hands-free device. Elective cardioversion has been done under conscious sedation without any adverse effects, but the usual technique is to use a sleep dose of propofol following pre oxygenation. One can use a facemask or maintain the airway with an LMA. If there is any serious doubt about cardiovascular performance or reserve, an arterial line should be given consideration, but this is a short procedure and the cardiac output should improve with the restoration of sinus rhythm. If the patient has a pacemaker in situ or an implantable cardiac defibrillator, we should place the paddles as far away as possible from the device and preferably in the anterior–posterior position.
IF THE PATIENT DOES NOT GET CARDIOVERTED, WHAT SHOULD YOU DO?
Try a period of 4–6 weeks of medical therapy and anticoagulation. If the patient is still in AF, then a further trial of DCC is reasonable. If a second DCC is unsuccessful, then rate control is the next step to improve symptoms and reduce ventricular failure.
