
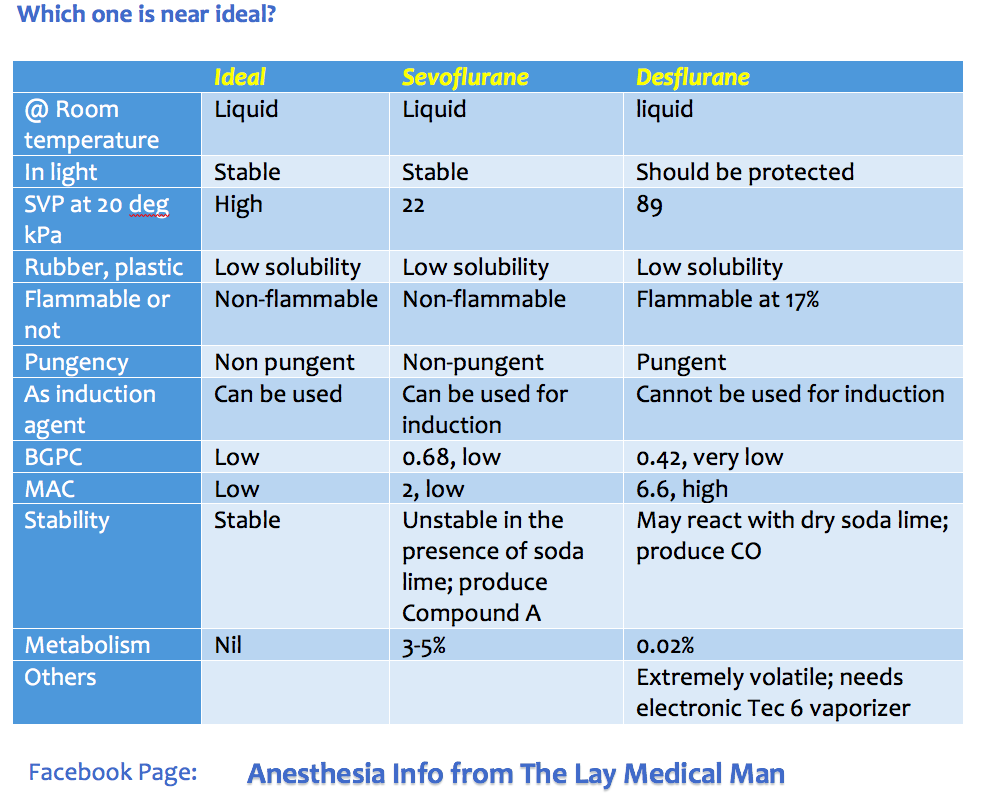
Sevoflurane vs Desflurane



ANTIPLATELET DRUGS
INHIBITORS OF Tx A2 PATHWAY
ASPIRIN: Aspirin inhibits TXA2 synthesis by irreversibly acetylating cyclooxygenase-1
INHIBITORS OF ADP RECEPTOR P2Y12
CLOPIDOGREL: Clopidogrel is a thienopyridine that irreversibly inhibits P2Y12 on the platelet surface. It’s a prodrug and must be metabolized in the liver to generate the active metabolites that inhibit the ADP receptor. The limitations are that it’s anti platelet action has a ceiling effect, and some patients are resistant to the effects of clopidogrel. Should be stopped at least 7 days prior to the surgery.
PRASUGREL: Prasugrel is also a thienopyridine that requires hepatic metabolism to generate active metabolites. But as this metabolism is more efficient than that of clopidogrel, prasugrel produces more rapid, more consistent, and more potent inhibition of ADP-induced platelet aggregation than clopidogrel does. Furthermore, the polymorphisms in CYP2C19 and CYP2C9 that limit the effectiveness of clopidogrel do not affect . Should be stopped at least 7 days prior to the surgery.
CANGRELOR: Cangrelor, which is administered intravenously, has a rapid onset and offset of action.
TICAGRELOR: Ticagrelor is an orally active inhibitor of P2Y12 that provides more rapid and complete antiplatelet effect than clopidogrel. Should be stopped at least 5 days prior to the surgery.
TICLOPIDINE: One potential side effect of ticlopidine therapy is neutropenia. Should be stopped at least 14 days prior to the surgery.
PHOSPHODIESTERASE INHIBITORS:
DIPYRIDAMOLE AND CILOSTAZOL: cAMP serves as an intracellular signal to suppress platelet activation and subsequent aggregation. By inhibiting phosphodiesterase, dipyridamole and cilostazol increase the levels of cyclic adenosine monophosphate. The risk of bleeding with these agents appears to be low.
GPIIb/IIIa ANTAGONISTS
ABCIXIMAB: Synthetic antibody which binds strongly to glycoprotein IIb/IIIa receptors (on platelets) thereby blocking the binding of fibrinogen (to platelets) thereby preventing the cross-linking of platelets which is needed for strengthening of the clot. It has the greatest affinity for GP IIb-IIIa among the GP IIb-IIIa antagonists. The prolonged platelet-bound half-life of abciximab accounts for both its prolonged effect on platelet function after termination of infusion and the gradual return of platelet function thereafter. Because the circulating pool of abciximab is bound to platelets, the effects of the agent can be reversed with the transfusion of platelets.
EPTIFIBATIDE AND TIROFIBAN: Both eptifibatide and tirofiban are synthetic antagonists of GP IIb/IIIa receptor.
GP IIb-IIIa antagonists are likely to be of greatest value when short term intense antiplatelet therapy is indicated, such as during PCI
ANTICOAGULANT DRUGS
THROMBIN INHIBITORS
DABIGATRAN ETEXILATE: Dabigatran etexilate, a prodrug, is an oral, direct thrombin inhibitor. Bioavailability is 6%. It can be administered as twice daily dosing. The drug is dialyzable. The effect can be monitored with ecarin clotting time or thrombin time. Dabigatran should be stopped 24-48 hours prior to any surgery. Idarucizumab, a monoclonal antibody, can reverses effects of dabigatran.
ARGATROBAN
It is intravenously administered reversible and a direct thrombin inhibitor approved for the management of acute HIT (type II). Advantages or uniqueness over other thrombin inhibitors includes its elimination through the liver (indication in compromising renal dysfunction) and short elimination half-life (35–40 min) that reveals normalization of aPTT in 2–4 h following discontinuation. However, dose reduction should be considered in critically ill patients and those with heart failure or impaired hepatic dysfunction.
HIRUDINS: LEPIRUDIN AND BIVALIRUDIN
These recombinant hirudins are first-generation direct thrombin inhibitors. They are administered by parenteral route, can accumulate in renal insufficiency and should be monitored using aPTT and ecarin clotting time (ECT).
DIRECT FACTOR Xa INHIBITORS
RIVAROXABAN AND APIXABAN : Are oral factor Xa inhibitors used in VTE prophylaxis. Effect can be monitored with anti Xa activity assay. Prothrombin Complex Concentrate may be used to reverse the effect. They should be stopped 48-72 hours prior to any surgery. An interval of 3 days should be kept before regional anesthesia.
IDRABIOTAPARINUX: is an indirect inhibitor of factor Xa that catalyzes the formation of irreversible antithrombin–factor Xa complexes. Idrabiotaparinux is thus the only one of the new oral or long-acting parenteral anticoagulants to have a specific antidote i.e. Avidin.
FONDAPARINUX: Is administered parenterally. It selectively inhibits factor Xa. It is licensed for use in thromboprophylaxis in medical patients and in patients undergoing major lower limb orthopedic surgery or abdominal surgery. After prophylactic dose at least 36-42 hours should elapse before performing central neuraxial blockade, whereas with therapeutic doses, one should avoid central neuraxial block.In patients with Heparin-induced thrombocytopenia (HIT), fondaparinux is the preferred agent. Prothrombin Complex Concentrate may be used to reverse the effect.
DANAPAROID
Danaparoid is an indirect factor Xa inhibitor with coagulation effects through antithrombin-mediated inhibition of factor Xa. It is a glycosaminoglycan mixture containing 84% heparin sulfate, dermatan sulfate, and chondroitin sulfate resulting in 10% incidence of HIT. It has a long elimination half-life of 22 h that could be prolonged with renal insufficiency. There is no antidote, coagulation monitoring can be done by measuring anti-Xa activity. It cannot be hemofiltered, but can be removed using plasmapheresis. It is used as an alternative in patients with HIT.
LMWH
e.g.: ENOXAPARIN, DALTEPARIN, TINZAPARIN
LMWH has an average molecular weight of 2000–10,000 daltons with a greater ability to inhibit factor Xa, than thrombin. It has a more predictable dose response curve and is administered at fixed dose, based on total body weight. LMWH has 100% bioavailability and reaches peak levels 2–4 h after S/C administration. It has a half-life of 3–4 h, and is eliminated primarily via renal clearance, necessitating dose reduction in patients with renal insufficiency. Factor Xa levels are used to monitor the effects of LMWH; ideally, factor Xa levels should be obtained 4 h after the administration of LMWH. Properties of LMWH differ from UFH in the following ways:
THROMBOLYTICS/FIBRINOLYTICS
ALTEPLASE, TENECTEPLASE, UROKINASE
Thrombolytic agents act by converting plasminogen to the natural fibrinolytic agent plasmin. Plasmin lyses the clots by breaking down fibrinogen and fibrin contained in the clot. Clot lysis elevates fibrin split/degradation products. Thrombolytic therapy will maximally depress fibrinogen and plasminogen for 5 h following therapy and remain depressed for 27 h.[25]
Initiation of thrombolytic therapy is contraindicated within 2 day following neuraxial/deep-PNB procedures and surgery; should perform assessments every 2 h for neurological deficits. The 2 day minimum is based on prolonged plasminogen depression for 27 h.
ANTIFIBRINOLYTICS
TRANEXAMIC ACID(TA) AND E-AMINOCAPROIC ACID: Tranexamic acid and ε-aminocaproic acid are synthetic antifibrinolytic amino acids (lysine analogues) that competitively block the lysine-binding site of both plasminogen and plasmin, therefore inhibiting each enzyme action.Plasmin can no longer bind to fibrin and can no longer degrade fibrin, and thus bleeding is reduced. Tranexamic acid and ε-aminocaproic acid have a small molecular weight and half life of about two to three hours. On a molar basis, TA is at least seven times more potent than EACA.
Clinically, tranexamic acid has been shown to increase the risk of seizures in patients undergoing cardiac surgery, largely when moderate and high doses (more than 10mg/kg) are used. Possible causal mechanisms include inhibition of GABA-A and glycine inhibitory receptors leading to stimulation of excitatory pathways, as well as an increased susceptibility of cardiac patients to postoperative seizures due to emboli introduced during surgery.
DOSE TA : 1g or 10mg/kg IV pre-incision bolus; maintenance: 1g iv over 8 hours (trauma) 1-5 mg/kg/hour (cardiac, ortho surgeries)
APROTININ: Aprotinin (a non-specific serine protease inhibitor) inhibits plasmin in a high dose and is the only agent shown to reduce the need for re-exploration.15 However, marketing of the drug was suspended in November 2007 after preliminary results of the BART trial, which showed an increasing mortality trend relative to the lysine analogues.
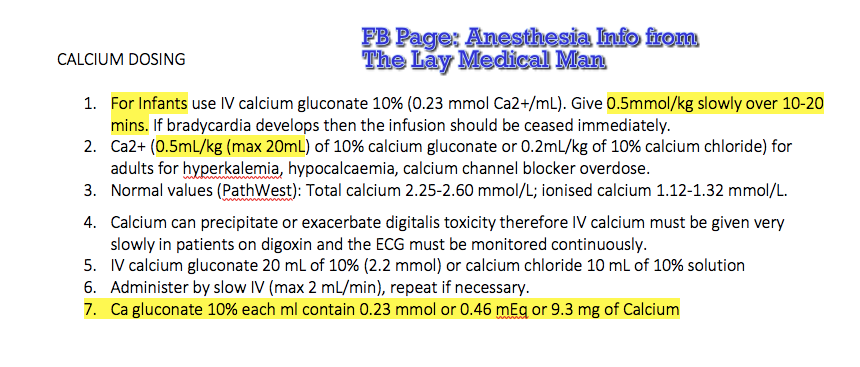
Plasma calcium < 2.2 mmol/L
Normal values: Total calcium 2.25-2.60 mmol/L; ionised calcium 1.12-1.32 mmol/L
HYPOCALCAEMIA CAUSES:
Decreased parathyroid hormone
Decreased Vitamin D activity (e.g. intestinal malabsorption, liver disease, CRF)
Increased calcium loss (e.g. chelating agents, calcification of soft tissues)
Decreased ionised calcium (e.g. alkalosis)
CLINICAL FEATURES:
ECG:
QTc prolongation by prolonging the ST segment
Torsades de pointes and atrial fibrillation in severe cases
NB: The corrected QT interval (QTc) is taken as the time between the beginning of the QRS complex and the end of the T wave, it is less than 440 ms in men and 460 ms in women. Severe hypocalcaemia (less than 1.9 mmol/L) may cause a prolongation of the QTc. A QTc greater than 500 ms is associated with an increased risk of Torsades de Pointes.
TREATMENT:
PARACETAMOL:
Central action via COX 3 inhibition which is associated with decreased brain PGE2 levels. It also modulates endogenous cannabinoid system
MORPHINE:
Morphine work by stimulating presynaptic Gi-protein-coupled MOP and KOP opioid receptors. Binding of the ligand causes the following events:
> Closure of voltage-gated Ca2+ channels
> Decreased cAMP production
> Stimulation of K+ efflux from the cell
> Hyperpolarisation of the cell membrane.
> This leads to decreased excitability of the cell and therefore decreased neurotransmitter release and pain transmission

N.B. Corrections:
LMWH inhibits factor Xa (Not XIa)