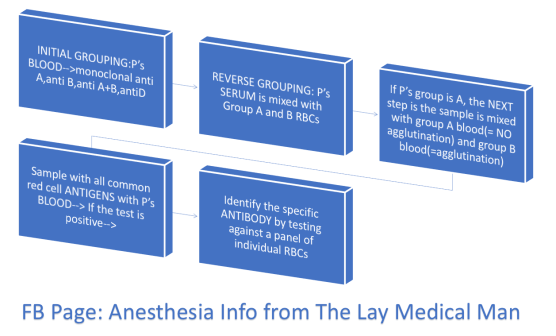
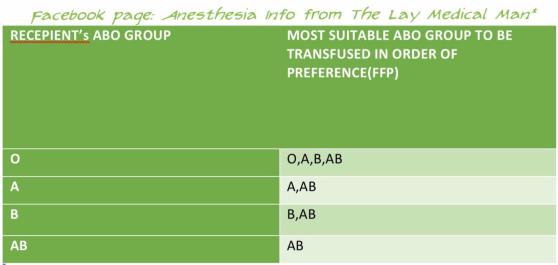
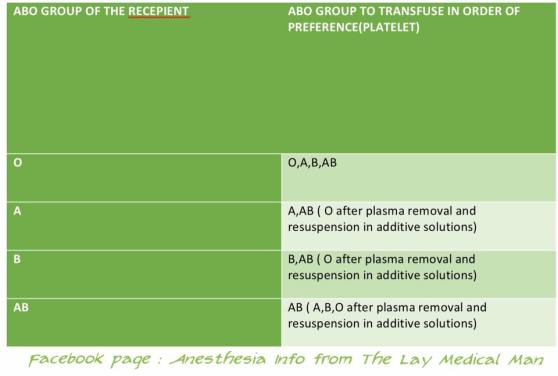
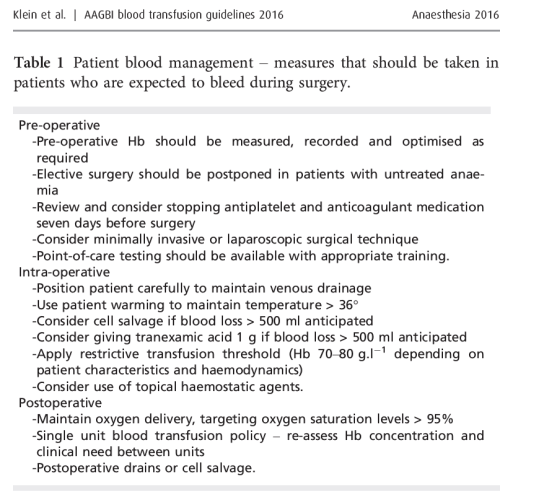
During routine epidural or spinal anaesthesia, accidental puncture of epidural veins occurs in 1–18% of patients
The incidence of hematoma after epidural techniques is estimated to be in the order of 1:150,000 after epidural placement and 1:220,000 after spinal injection in the general population
removal of epidural catheters posed an equal risk to insertion ( Van- dermeulen et al)
Surgery on spinal haematoma should ideally be performed within 8–12 h of the identification of symptoms in order to improve the chances of recovery.
The overall risk of death in those having general anaesthesia for caesarean section was quoted in 2007 as being just over 1:25,000.
The levels of factors VII, VIII and fibrinogen increase and those of anticoagulation factors decrease, causing augmented coagulation and decreased fibrinolysis in pregnancy.
There is no evidence to support routine full blood count (FBC) or coagulation tests in women before the performance of a regional block in those who have had
normal FBC results
no bleeding history
no signs or symptoms of liver disease
no signs or symptoms of pre-eclampsia, abruption or clinical signs of disseminated intravascular coagulation
no recent anticoagulant treatment.
In women with known thrombocytopaenia, a Full Blood Count (FBC) should be checked within 24 h of a regional procedure.
In women with mild to moderate pre-eclampsia, the course of the disease can be unpredictable and so FBC be checked within 6 h. In addition, coagulation tests should be performed if platelets are <100000/mcL or if there is abnormal liver function.
In severe disease, FBC and clotting should be checked immediately before a procedure, as platelet levels in particular can decline rapidly.
Women with pregnancy-induced hypertension alone do not require an FBC before a regional procedure
Activated partial thromboplastin time ratio (APTTR) and international normalised ratio (INR) are slightly decreased in late pregnancy.
In a patient who receives LMWH, if he/she is simultaneously taking NSAID+Aspirin, there is an increased risk if last dose of LMWH is between 12-24 hours; it further increases if last dose is <12 hours
In patients with pre-eclampsia and platelet count between 75000-100000/mcL, there is an increased risk even if coagulation tests are normal; but it increases further if the counts has not been stable (=decreasing platelet count)
#obstetrics , #anesthesia , #coagulation , #anaesthesia
Reference: Abnormalities of Coagulation and Obstetric Anaesthesia, Hilary Swales, AAGBI Core Topics in Anaesthesia 2015