Awake Craniotomy: Decoding a misnomer!
TIVA IN NEUROANAESTHESIA
SAFE & APPROPRIATE ADMINISTRATION OF TIVA
Arterial Blood Gas Analysis- Combined Copenhagen Physico-chemical Approach
AEP Episode 2: Pseudomonas infections and their treatment
AEP Episode 1: SSI and prevention of infection in the ICU
Anaesthesia Exam Podcast: Crystalloids vs Colloids
BALANCED SALT SOLUTIONS
- Intravenous “balanced” solutions include crystalloids and colloids with minimal effect on the homeostasis of the extracellular compartment, and in particular on acid–base equilibrium and electrolyte concentrations. These are fluids which are able to leave the plasma pH unchanged after its administration.
- There are two main categories of balanced solutions : (1) fluids causing a minimal effect on acid–base equilibrium, having an electrolyte content with an in vivo strong ion difference (SID), i.e., the SID after metabolism of the organic anion, close to 24–29 mEq/L; (2) fluids having a normal or sub-normal Cl−content(Cl− ≤ 110 mEq/L).
- The ideal balanced solution should have an in vivo SID equal to the baseline concentration of HCO3−. If the SID of the infused fluid is greater than plasma HCO3−, plasma pH will tend toward alkalosis; if the SID of the infused fluid is lower than plasma HCO3−, plasma pH will tend toward acidosis, as it is always the case for NaCl 0.9%.
- An isotonic balanced solution leaving unaltered acid–base equilibrium (i.e., with an SID close to 24 mEq/L) will necessarily have a Cl− content > 110 mEq/L (as in Sterofundin-ISO).
- In contrast, a fluid with an SID of 24 mEq/L and a lower Cl−content will necessarily be slightly hypotonic (as with Lactated Ringer’s). Finally, an isotonic fluid with a low Cl−content will necessarily have a higher SID (as with PlasmaLyte), with a consequent alkalizing effect.
- Chloride-rich NaCl 0.9% causes a higher dose-dependent degree of acidosis and hyperchloremia, which possibly favors the contraction of vascular smooth muscles, potentially leading to a reduced renal perfusion.
- Hyperchloremia may cause increased tubule-glomerular feedback and decreased renal cortical perfusion. NaCl 0.9%, being slightly hypertonic, likely causes an increased incretion of arginine vasopressin.
- These effects can conceivably contribute to the slower renal excretion of NaCl 0.9% as compared to balanced solutions. Indeed, more fluid will be retained in the interstitial space, with the consequent propensity to cause more edema.
- Therefore, the use of balanced solutions, particularly in patients that potentially need a significant amount of intravenous fluids, seems to be a reasonable pragmatic choice.
- On the contrary, saline may be an intuitive choice for patients with hypovolemic hyponatremia or hypochloremic metabolic alkalosis.
- In any other settings, the most important reason to choose NaCl 0.9% over balanced solutions is likely economic in nature. Therefore, the patient’s serum chloremia is an important factor to determine the appropriate type of fluids
Bridging Anticoagulation
Bridging anticoagulation consists of the substitution of a long-acting anticoagulant (usually with warfarin) for a shorter-acting anticoagulant (usually LMWH) to limit the time of subtherapeutic anticoagulation levels and minimize thromboembolic risk. Despite the growing evidence about the limited to nonexistent benefits of bridging therapy, it is still being used on a case-by-case basis. Clinical scenarios that may benefit from bridging therapy are those involving patients with high thromboembolic risk. In several guidelines, the following scenarios have been proposed:
- The patient with a mechanical heart valve: Mitral valve replacement, aortic valve replacement with additional risk factors (stroke, TIA, cardioembolic event, or intracardiac thrombus), more than 2 mechanical valves.
- Patients with stroke, episode of systemic emboli, or VTE during the last 3 months. Patients presenting with a thromboembolic event after interruption of chronic anticoagulation therapy or those presenting with VTE while on therapeutic anticoagulation.
- Patients with atrial fibrillation and CHA2DS2VASc score > 5 plus additional cardiovascular risk factors (rheumatic valve disease, stroke, or systemic embolism within the last 12 weeks). A CHA2DS2VASc score > 6 with or without additional risk factors.
- Patients with recent coronary stenting (within the previous 12 weeks)
How to bridge?
During the preoperative period:
- Discontinue warfarin five days before surgery.
- Three days before surgery, start subcutaneous LMWH or unfractionated heparin (UFH), depending on the renal function of the patient at therapeutic doses.
- Two days before surgery assess INR, if greater than 1.5 vitamin K can be administered at a dose of 1 to 2 mg.
- Discontinue LMWH 24 hours before surgery or 4 to 6 hours before surgery if UFH.
During the postoperative period:
- If the patient is tolerating oral intake, and there are no unexpected surgical issues that would increase bleeding risk, restart warfarin 12 to 24 hours after surgery.
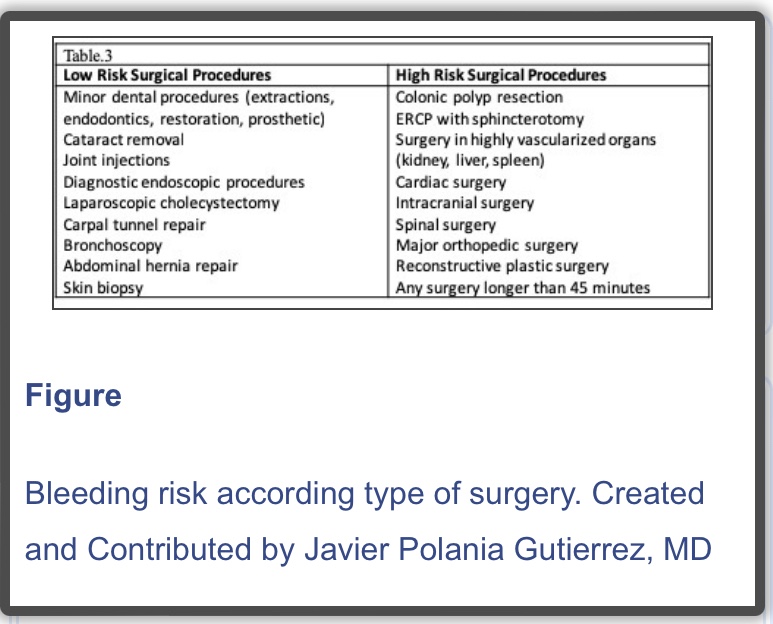
- If the patient received preoperative bridging therapy (high thromboembolic risk) and underwent a minor surgical procedure, resume LMWH or UFH 24 hours after surgery. If the patient underwent a major surgical procedure, resume LMWH or UFH 48 to 72 hours after surgery.
- Always assess the bleeding risk and adequacy of homeostasis before the resumption of LMWH or UFH
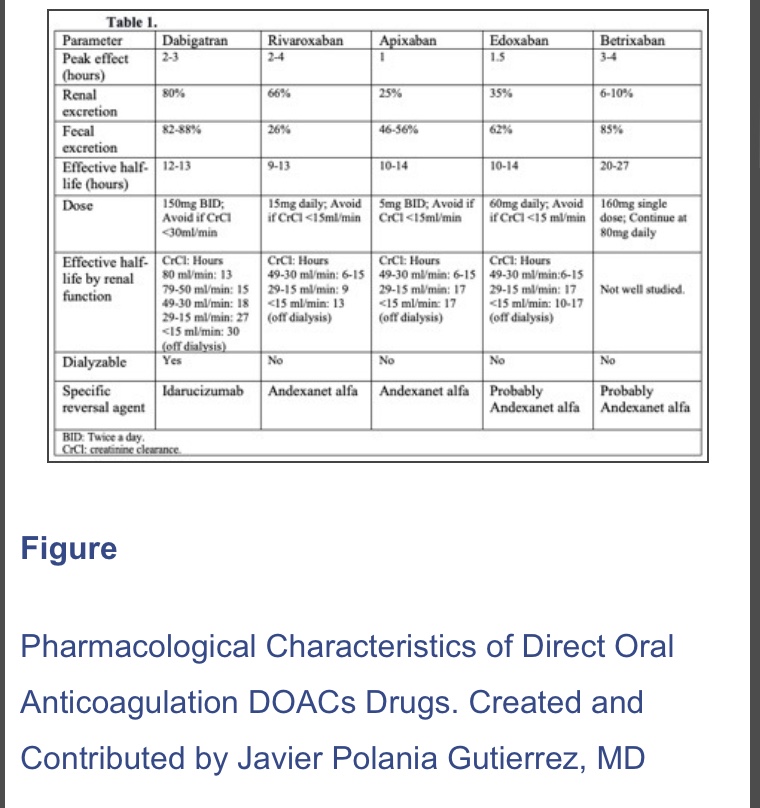
N.B. : In 2019, a new strategy was published in the PAUSE study, a prospective clinical trial evaluating a standardized approach for perioperative management of DOACs. The interruption scheme used in this study was simple. For high bleeding risk procedures, rivaroxaban, apixaban, and dabigatran were suspended 48 hours before surgery in patients with CrCl>50 ml/min. If the renal function was compromised (CrCl< 50 ml/min), these drugs were interrupted for four days before surgery. For low bleeding risk procedures, rivaroxaban, apixaban, and dabigatran were interrupted 24 hours before surgery in patients with CrCl>50 ml/min. If the renal function was compromised (CrCl <50 ml/min), drugs were suspended two days before the procedure. Regardless of renal function, all drugs were reinitiated at 48 hours for high bleeding risk surgical procedures and 24 hours for low bleeding risk procedures. The 30-day postoperative rate of major bleeding was 1.35% (95% CI, 0%-2.00%) and rate of arterial thromboembolism of 0.16% (95% CI, 0%-0.48%). However, more studies are needed in patients with high surgical bleeding risk, before implementing this in regular clinical practice
Reference:
Perioperative Anticoagulation Management – StatPearls – NCBI by Polania Gutierrez JJ, Rocuts KR. · 2021
https://www.ncbi.nlm.nih.gov/books/NBK557590/