Anatomy of lumbar plexus?
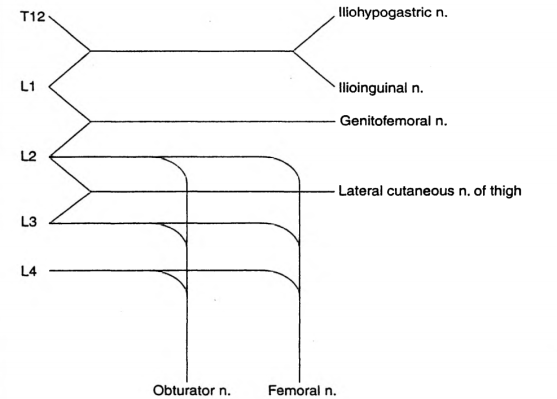
The lumbar plexus is formed by the anterior primary rami of L1 to L4 (Sometimes T12 also may contribute)
L1 forms the iliohypogastric and ilioinguinal nerves
It also gives a contribution to the formation of the genitofemoral nerve
L2 forms the lateral cutaneous nerve of thigh, along with L3
The obturator and femoral nerves are formed by contributions from L2,L3,L4
What are the indications?
Surgeries involving hip/thigh/upper leg/trauma
In conjunction with sciatic nerve or sacral plexus block
Cancer pain arising from hip or upper femur
Sympathetic block also helps in ischemic pain and CRPS
Which approach will you use?
I will use the 3 in 1 approach that aims to block the femoral, obturator and lateral cutaneous nerves with a low anterior approach. The patient is in the supine position.I will use a nerve stimulator. Using a 50 mm insulated needle, I will puncture the skin at a point 1 cm lateral to the femoral pulse and 2 cm below the inguinal ligament, at 45 degrees to skin and directed proximally and parallel to the femoral artery. The endpoint is a quadriceps twitch, which occurs at a depth of 30-50 mm. I will press distal to the injection to enhance the spread of the drug proximally. I will block the lateral cutaneous nerve separately by injecting 10 ml of local anesthetic at a point 2 cm inferior and medial to the ASIS. Other approaches are psoas compartment block and fascia iliacus block
How does the lumbar sympathetic block differ from the lumbar plexus block?
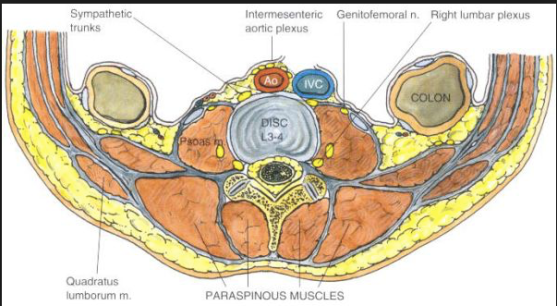
The lumbar sympathetic nerves lie anterolateral to the vertebral body, whereas the somatic nerves lie posterior to the psoas muscle and fascia. The sympathetic chains have anteriorly, aorta on the left and IVC on the right. The aim is to deposit local anesthetic around the nerves from L2 to L4 either with a single injection at L3 or 3 separate injections at L2, L3 and L4. It is used in lower limb ischemia, CRPS, phantom limb, urogenital pain etc
How to do the lumbar sympathetic block?
Patient should be positioned lateral with the side to be blocked up. A point is marked 8 cm from the midpoint of the spinous process of the desired vertebra. Its done under image guidance. A 12 cm 22 G needle is inserted at 45 degree angle directed medially towards the vertebral body which lies at a depth of around 8 cm; if the needle hits the bone at 4-5 cm depth it is likely to be the transverse process and should be redirected cranially or caudally to pass over it. Once the needle hits the vertebral body, it should be redirected slightly antero-laterally till we feels the pop-off of passing the psoas fascia. Then the local anesthetic mixed with radiographic contrast is injected which should form a band around the desired vertebral bodies. If the contrast disappears very quickly, it may be in a vessel. Otherwise, it can go into the psoas muscle or retroperitoneal tissue. Also we should take lateral and anteroposterior images to confirm the correct position.
Complications of lumbar sympathetic block?
Local anesthetic toxicity due to injection into aorta or IVC
Profound motor block or permanent paralysis due to intrathecal injection
Profound hypotension: good iv access and access for resuscitation equipments are a must
Post sympathectomy pain
Ureteric injury, ejaculatory failure