MECHANISM OF ACTION:
- Anti Diuretic Hormone (ADH) stimulates V2 receptors on collecting ducts, which increases adenylate cyclase activity. This causes fusion of pre-formed water channels on the apical membrane resulting in increased permeability of the collecting ducts to water.
ADH is secreted in response to:
- Hyperosmolarity: detected by osmoreceptors in the hypothalamus, outside the blood–brain barrier. Osmoreceptors then stimulate thirst
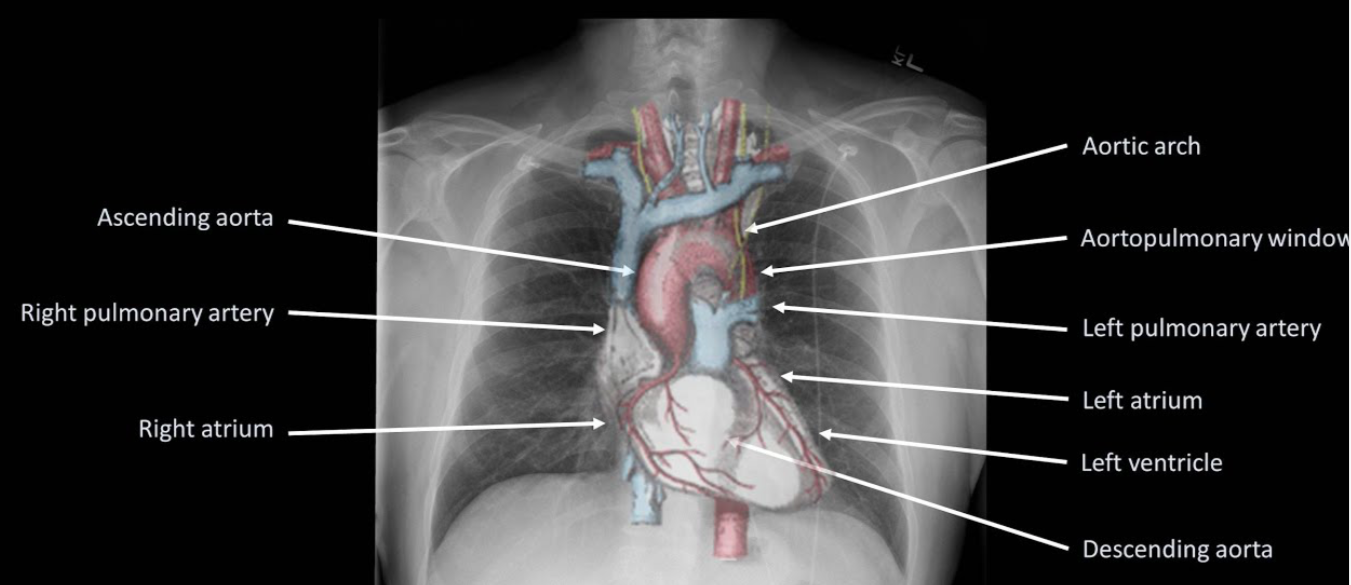
Volume depletion (ECF) detected by low-pressure baroreceptors in great veins, atria and pulmonary vessels, and high-pressure baroreceptors in the carotid sinus and aortic arch
Angiotensin II (AGII)
- Other: pain, exercise, stress, emotion, nausea and vomiting, standing, nicotine, morphine, barbiturates, carbamazepine.
EFFECTS:
It regulates Total Body Water (TBW).
The specific renal effects of ADH on water balance include:
- Increased water permeability in cortical collecting tubule (V2 receptors)
- Increased water and urea permeability in medullary collecting tubule
- Increased retention of water
- Reduced urine volume
OTHER EFFECTS
- It stimulates thirst
- Release of factor VIII by the endothelium
- Platelet aggregation and degranulation
- Arteriolar vasoconstriction
- Glycogenolysis in the liver
- Brain neurotransmitter
- Secretion of ACTH from the anterior pituitary gland.

VASOPRESSIN RECEPTOR AGONISTS
Arginine-Vasopressin: A V receptor agonist is a potent alternative to vasoconstrictors in the treatment of fluid and catecholamine-refractory septic shock
Terlipressin is a selective V1 receptor agonist and may be more potent than arginine-vasopressin in restoring catecholamine refractory septic shock
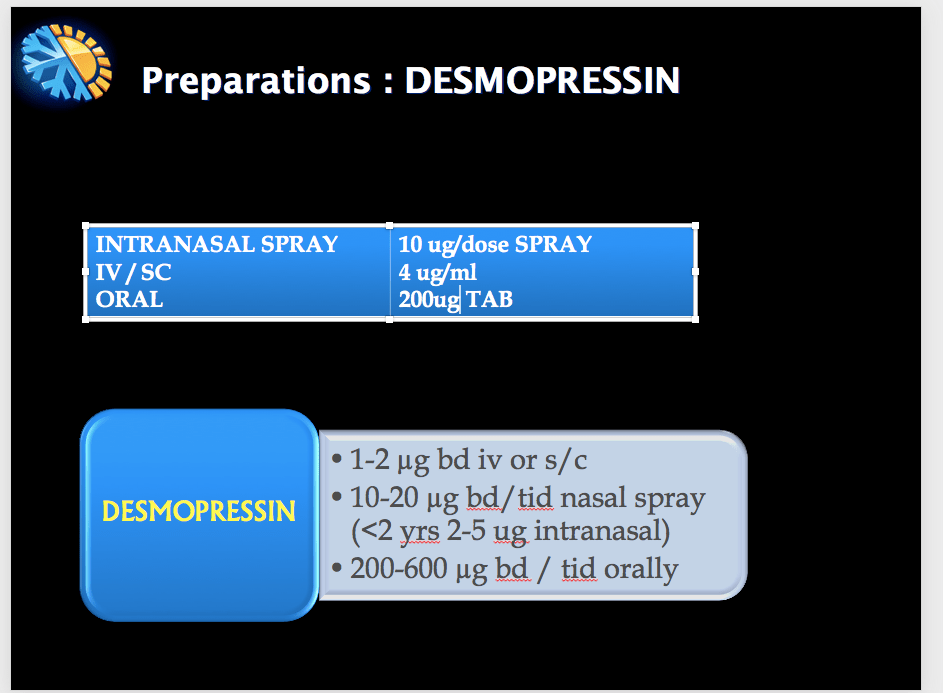
Desmopressin (1-deamino-8-D-arginine vasopressin) is a synthetic vasopressin analog, that acts as an agonist at V1B and V2 receptors. Exhibits antidiuretic and haemostatic properties. So also used in the management of some forms of hemophilia and von Willebrand’s disease

ANTAGONISTS
Vaptans act by inhibiting vasopressin’s action on all the 3 types of receptors. e.g. Conivaptan (unselective), Tolvaptan (V2 selective). Used in the treatment of euvolemic (eg SIADH) and hypervolemic (CHF) hyponatremias, nehrogenic DI, cirrhosis, PCKD etc