1. PYLORIC STENOSIS (PROTOTYPE CASE; ANY OTHER CASE, MAKE THIS A TEMPLATE AND ADD SPECIFIC POINTS RELEVANT FOR THAT CONDITION)
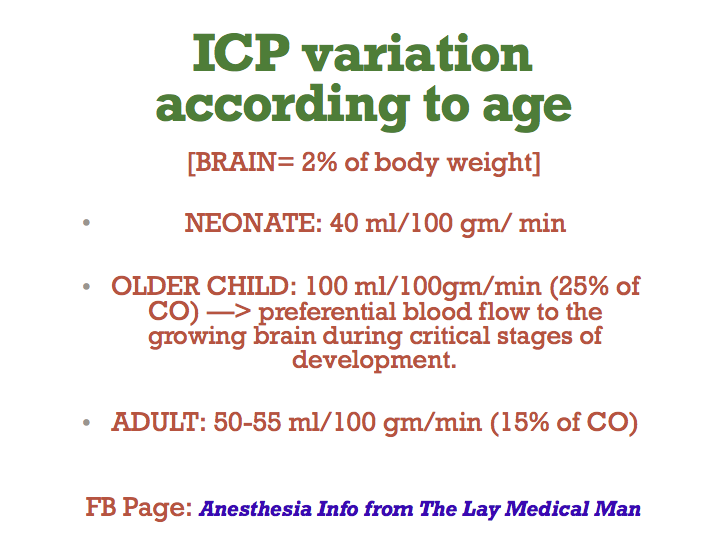
Most common cause of intestinal obstruction in infancy
Due to hypertrophy of circular pyloric muscle
Presentation: 4-6 weeks of age
Persistent projectile vomiting
There is obstruction at the level of pylorus: so bicarbonate rich fluid from intestine cannot mix with gastric secretions
So the vomiting causes metabolic alkalosis and hypokalemia due to loss of acidic gastric juice alone
The large bicarbonate load is in excess of kidney’s absorptive capacity and the urine becomes alkaline initially
Later when the fluid and electrolyte loss result in dehydration, the renin angiotensin system is activated and result in aldosterone secretion which tries to preserve sodium at the expense of K and Cl ions
This result in production of paradoxical acidic urine and worsening of metabolic alkalosis and hypokalemia
An attempt to compensate it through hypoventilation is initiated; but it will be insufficient and also this will trigger the hypoxic drive!
Hypoglycemia, hemoconcentration, mild uremia and unconjugated hyperbilirubinemia may be seen.
ANAESTHETIC CONCERNS
Pyloric stenosis is not an emergency
The DYSELECTROLYTEMIA and ACID BASE IMBALANCE should be corrected before taking up for surgery.
Increased chance for regurgitation, altered physiology and anatomy, altered drug dosages, difficult venous access, anxious parents are other concerns
There should be an experienced paediatric anaesthesiologist for the conduct of anaesthesia
P.A.C. AND EVALUATION
LOCATION: Needs resuscitation in a paediatric ICU or ward
INVESTIGATIONS: CBC, Blood sugar, RFT, LFT, group and hold, serial ABGs to know the effectiveness of resuscitation and decide on the dose of K administration. 3 mmol/kg/24 hour potassium should be added to maintenance fluids
HISTORY: From the parent; vomiting frequency, amount of feeds taken, diarrhoea, frequency of wetting of the nappy, fever, altered sensorium
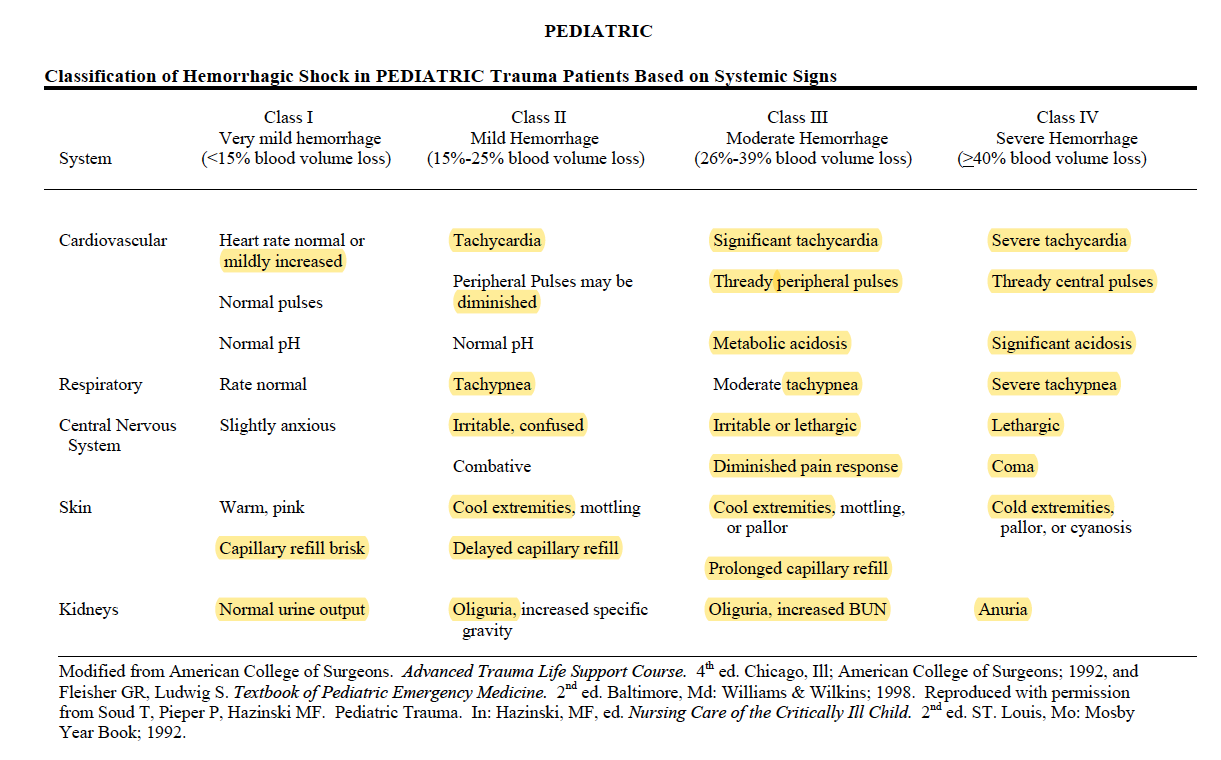
EXAM: ?Dry mucus membrane ?Dry eyes (5% dehydration) ?Sunken fontanelle ?cool peripheries ?oliguria(10%) ?Hypotension ?Tachycardia ?Altered Sensorium (10%) ?prolonged capillary refill time
Naso Gastric tube insertion and 4 hourly aspiration of residue
FLUID RESUSCITATION:
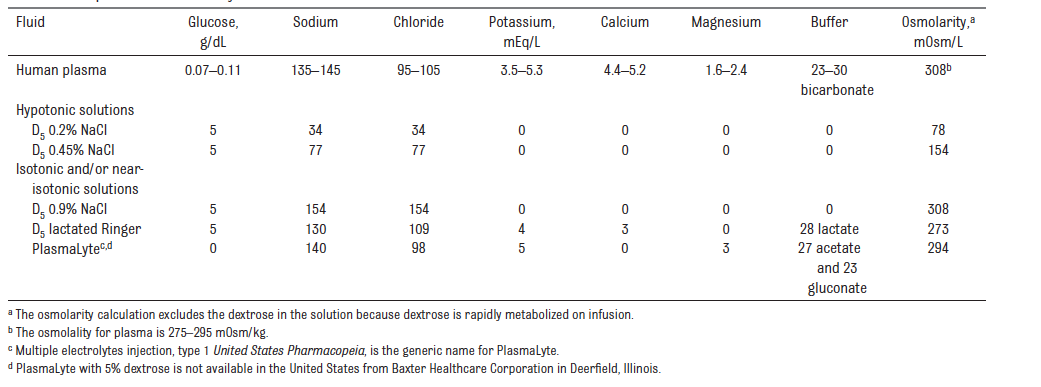
- For fluid resuscitation : use glucose‑free crystalloids that contain sodium in the range 131–154 mmol/litre, with a bolus of 20 ml/kg over less than 10 minutes. PlasmaLyte, 0.9% Saline ad Ringer Lactate
MAINTENANCE FLUIDS:
- Calculate routine maintenance IV fluid rates using the Holliday–Segar formula (4 ml/kg/hr for the first 10 kg of weight, 2 ml/kg/hr for the next 10 kg and 1 ml/kg/hr for the weight over 20 kg).
- This solution would preferably be enriched with glucose 5% (50 ml glucose 50% in 500 ml fluid) in order to provide an adequate caloric supply as recommended (4 to 8 mg glucose/kg/min). In addition, the osmolarity of such a solution makes it possible to be administered on a peripheral venous access.
- Intraoperative: also will assess for blood loss; the maximum speed of transfusion should be 10ml/kg/hour (if no cardiac failure). Estimated blood volume x ( Desired Hct – Current Hct / Hct of the RBC ) will give the volume of PRBC to be transfused. (N.B.: As a general guide in laparotomies 10 ml/kg/ hour would be needed to compensate for evaporative losses). FFP if needed: 10-15 ml/kg
TARGETS:
- Normovolemia
- 1-2 ml/kg/hr of urine OR at least 2 wet nappies
- Chloride > 95 mmol/L
- pH <7.5
- Base excess <6 mmol/L or bicarbonate < 30 mmol/L
CONDUCT OF ANAESTHESIA
- Ensure that the child is adequately resuscitated
- Procedure may take 30-60 mins
- Paediatric anaesthetist
- Ensure the availability of drugs-equipments for anaesthesia induction, maintenance and resuscitation
- Attach monitors: ECG, SpO2 and NIBP(AAGBI standards)
- 4 quadrant aspiration via Ryles tube
- Preoxygenation for 3 mins
- Modified rapid sequence induction with cricoid pressure
- Propofol, Fentanyl and succinyl choline/ rocuronium 1 mg/kg
- Straight bladed laryngoscope. Insert an uncuffed ETT of size 3.5 mm ID; I will also keep size 3 and 4
- Ensure proper placement: 5 point auscultation and checking capnography trace
- Release cricoid pressure and secure the tube
- Hand ventilate the child before connecting to a ventilator (ventilation guided by ETCO2 values)
- Paediatric mode; Pressure Controlled Ventilation
- O2, Air and sevoflurane maintenance
- Can consider an epidural for intra and postoperative analgesia (C/I Sepsis, coagulopathy)
- Local anesthetic can be infiltrated by the surgeon; Ropivacaine or levobupivacaine; dose < 2mg/kg
- Paracetamol iv (child <10 kg–> 7.5 mg/kg 6 hourly; max 30 mg/kg/day) OR
- Paracetamol suppository 30 mg/kg loading dose followed by 20 mg/kg 6-8 hourly: max 60 mg/kg/day; continue postoperatively
- Fentanyl 1microgram/kg iv boluses hourly is less cumulative and more predictable than morphine
- If well hydrated with good renal perfusion: NSAIDs Ibuprofen 3 mg/kg three times daily or diclofenac per rectal 1 mg/kg/dose suppositories
- Monitor urine output
- Antiemetics: ondansetron 0.15 mg/kg
- Extubation in lateral position
- Risk of post operative apnoea: SpO2 monitoring with apnoea alarm
- Regular and PRN analgesics in postoperative period
OTHER INTRAOPERATIVE CONCERNS
- Strategies to avoid excessive heat loss, optimal analgesia and depth of anaesthesia, optimal fluid therapy
- Constant monitoring of heart rate, oxygen saturation,EtCO2, blood pressure, body temperature, urine output
- Check blood sugar to avoid hypoglycemia
- To prevent hypothermia: Increase the ambient temperature of the OR prior to and during the surgery, body warmer, bubble wraps and plastic drapes to cover the body, cover the head, warm the iv fluids, HME to reduce heat loss from the respiratory system
PAIN EVALUATION IN PAEDIATRICS
- Evaluated with the support of the parents
- Using a combination of physiological and behavioural markers in neonates and infants
- e.g. facial expression, sleeplessness, cry, body movements, posture, increased clinginess, loss of appetite, screaming, reluctance to move, CVS & RS changes
- Neonate and Infant: Neonatal Pain Agitation and Sedation Score (N-PASS), Neonatal and Infant Pain Scale (NIPS), Face, Legs, Activity Cry and Consolatility scale (FLACC), Objective Pain Score
- Pre-school and school children can be evaluated by scales like Faces scale ( Happy to crying faces
- Adolescents: Visual Analogue Scale
POSTOPERATIVE CARE
- In a high dependency unit or Paediatric Intensive Care unit
- Will watch for respiratory depression and respiratory compromise, ?shock, pain levels, surgical complications, signs of loss of blood volume
- Send sample for full blood count and serum electrolytes
- Continue IV fluids
2.INTUSSUSCEPTION
- Small bowel telescoping; most common cause of intestinal obstruction in first year of life; other diseases like Meckel’s diverticulum, lymphoma etc can present as intussuception.
- HISTORY:Present with paroxysmal abdominal pain- child may hold legs to abdomen and red currant jelly like stools. Vomiting and dehydration can cause shock. Abdominal distension can compromise respiration. Infection, infarction,bleeding, perforation, Sepsis-shock etc also can occur.
- EXAMINATION:Abdomen-guarding. Sausage shaped mass on the right side.
- INVESTIGATION: Abdominal Ultra sound. X ray abdomen: ?obstruction ?perforation. Air enema can be therapeutic also but contraindicated if having perforation or peritonitis or if in shock.
- If in shock–> resuscitate and take for laparotomy. A
- ny repiratory compromise due to distension–> consider the need for mechanical ventilation
3.OESOPHAGEAL ATRESIA (OA) & TRACHEO OESOPHAGEAL FISTULA (TOF)
- Defective embryonic development of oesophagus and trachea
- Mostcommon types are 1. isolated OA with distal TOF 2. isolated OA and 3. isolated TOF
- PAC: ask for SYMPTOMS: Repeated episodes of coughing,chocking and cyanosis worsened by feeding, resitance to pass a nasogastric tube; evaluate for associated congenital anomalies- VACTERL: Vertebral Anal Cardiac Tracheo Esophageal Renal Limb anomalies; problems of prematurity- lung disease, retinal problems, impaired glucose regulation
- OPTIMISATION: The ligation of a TEF is urgent, but not emergent, except in the setting of respiratory insufficiency severe enough to require ventilatory support. Use IV fluids with glucose to avoid hypoglycemia and to achieve euvolemia. Use specialised suction called Replogle suction to clear the upper part of the oesophagus. Avoiding feeding. Upright positioning of the infant to minimize gastroesophageal reflux. Administration of antibiotic therapy to treat sepsis or aspiration pneumonia. Uncomplicated surgery can be done within 24 hours of birth, to reduce the chance of aspiration of pooled secretions.
- ANAESTHESIA TECHNIQUE (See above) Specific points for TOF: Under monitoring with pulseoximeter, suction the upper pouch–>Preoxygenation with 100 % O2; avoid vigorous bag and mask ventilation; else the air will pass via TOF into stomach causing distension and splinting of the diaphragm–> An awake technique with titration of small doses of fentanyl (0.2 to 0.5 mcg/kg) allows intubation of the trachea without excessive hemodynamic stimulation or depression. OR an inhalational anesthetic with or without muscle relaxation and with cautious, gentle positive pressure ventilation as needed (Ref: Smith’s anaesthesia for infants & children 8/e) –> place ETT–> allow child to breath spontaneously–>examine airway with a rigid bronchoscope and establish the exact anatomy–> replace and position ETT in a way to occlude the TOF–> once the ETT is placed in the correct position, NMBA can be given. Consider IBP and central venous access based on individual case. Child usually in Right lateral position; take care to maintain the iv access safely.
4.CONGENITAL DIAPHRAGMATIC HERNIA (CDH)
- Repair is not an emergency procedure. Usually delayed for 24-48 hours for the pulmonary resistance to come down. Baby should be resuscitated first. Delivery should take place as close to term as possible to achieve maximum lung maturity. ABG, Chest x-ray and Echo shold be done. Do an oroogastric suction to clear the part of the bowel in the chest. Intubatin and ventilation should aim to reduce barotrauma with smaller tidal volumes and permissive hypercapnea.
5.EXOMPHALOS & GASTROSCHISIS
- Due to failure of the migation of the gut into the abdominal cavity during fetal development, there is a herniation of the abdominal contents with a covering sac through a midline defect in the abdominal wall and other congenital anomalies may accompany. Whereas gastroschisis is an isolated anomaly where the gastric contents without any covering sac herniates through a defect in the abdominal wall but not in the midline.
- There is chance of infection and extensive loss of heat and moisture from the exposed bowel; so it should initially be covered with a non porous material and extreme priority shoud be given for the eveluating the fluid loss and replacement and also for establishing normothermia
- Primary closure or if respiratory compromise is not allowing it, a staged closure may be necessary
6. INGUINAL HERNIA / REGIONAL TECHNIQUES
-
Mask or IV induction with general anesthesia (with or without endotracheal intubation); spinal anesthesia as a primary anesthetic; caudal anesthesia or analgesia. Muscle relaxation is not necessary but may be a valuable adjunct for decreasing anesthetic requirements and providing optimal surgical conditions.
-
Equipment: Low compression volume anesthesia breathing circuit (circle absorption system vs. Mapleson D ). Monitoring: Standard noninvasive monitoring
- Maintenance: 1. Inhalation agent plus local infiltration or ilioinguinal-iliohypogastric block on the surgical field OR maintenance caudal analgesia, allow the avoidance of opiates. Neuromuscular blockade with nondepolarizing agent, if chosen. 2. Volume support with judicious amounts of crystalloids.(See 1.) Blood loss is minimal.
-
Emergence and Perioperative Care: 1. Vigilance regarding perioperative abnormalities of control of breathing: periodic breathing/apnea; laryngospasm/bronchospasm; bradycardia; hypoglycemia; continuously monitor with a pulseoximeter. 2. Pain management. (See:1)
Regional anaesthesia:
- SPINAL ANAESTHESIA: For neonates and infants, a 1 inch 22-gauge spinal needle is inserted at the L4-5 interspace. 0.5 to 0.6 mg/kg of either isobaric or hyperbaric bupivacaine will provide an average of 80 minutes of surgical analgesia for infants and young children who are less than 5 kg in weight. For infants or toddlers 5 to 15 kg, the dosage of hyperbaric bupivacaine or tetracaine is 0.4 mg/kg, and for children weighing more than 15 kg, the dosage of bupivacaine or tetracaine is 0.3 mg/kg . Isobaric ropivacaine has been studied in children for spinal anesthesia at a dosage of 0.5 mg/kg up to 20 mg.
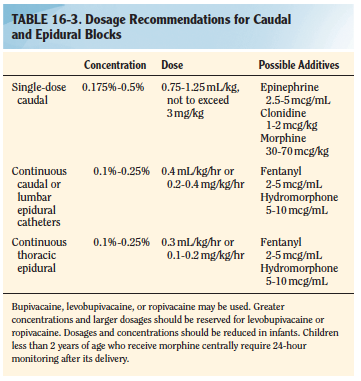
- CAUDAL BLOCK: The recommended concentration of bupivacaine for a singleshot caudal is 0.125% to 0.25%. An epinephrine dosage of 2.5 mcg/mL, or a concentration of 1:400,000 may be used as an additive for central blocks. Epinephrine will serve as a marker for intravascular injection and decrease systemic absorption of local anesthetic. Additionally, epinephrine may prolong the duration of a regional block. Preservative Free Ketamine, fentanyl, clonidine etc also can also be used as additives. See table: Smith 8/e
- ILIOINGUINAL /ILIOHYPOGASTRIC BLOCK: Can be given after induction, but before the incision; a blunt 22 or 25 gauge needle is inserted 1 cm superior and 1 cm medial to the anterosuperior iliac spine; the needle is initially directed posterolaterally to contact the inner superficial lip of the ileum, then withdrawn while injecting local anesthetic during needle movement. Once skin is reached, the needle is redirected toward the inguinal ligament (ensuring that the needle does not enter the ligament) and local anesthetic is injected after a pop is felt. Can perform under US guidance too. Can use 0.25 mL/kg of 0.5% levobupivacaine or 0.5% ropivacaine at 3 mg/kg. (Ref: Smith’s anaesthesia for infants & children 8/e)